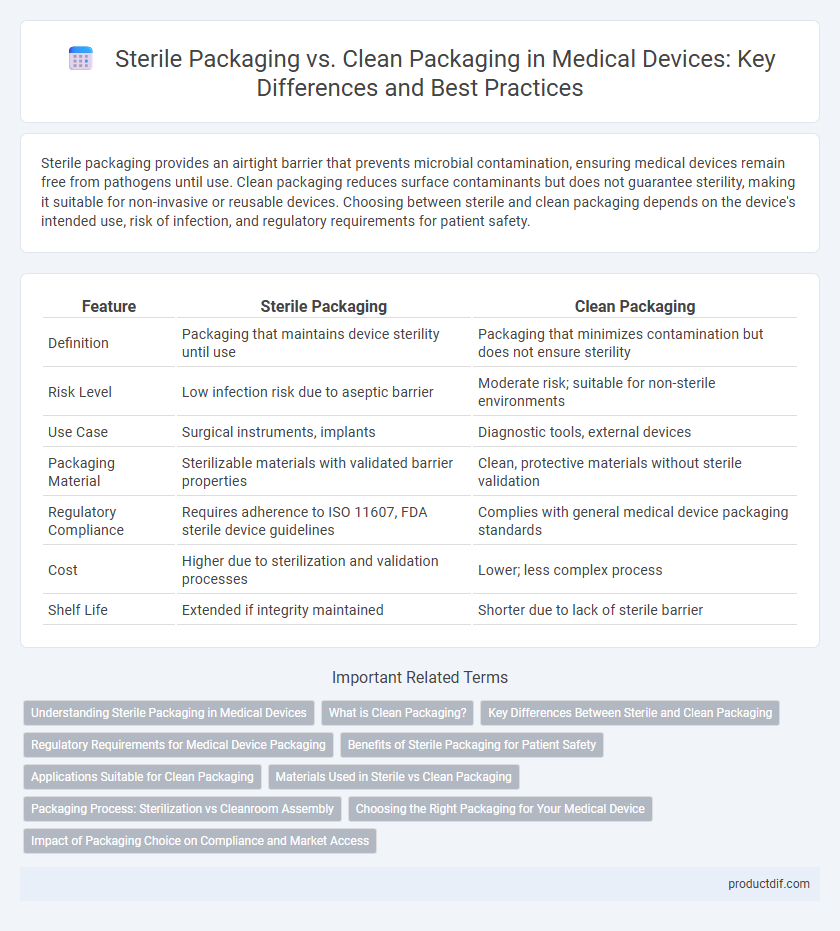
Sterile packaging provides an airtight barrier that prevents microbial contamination, ensuring medical devices remain free from pathogens until use. Clean packaging reduces surface contaminants but does not guarantee sterility, making it suitable for non-invasive or reusable devices. Choosing between sterile and clean packaging depends on the device's intended use, risk of infection, and regulatory requirements for patient safety.
Table of Comparison
| Feature | Sterile Packaging | Clean Packaging |
|---|---|---|
| Definition | Packaging that maintains device sterility until use | Packaging that minimizes contamination but does not ensure sterility |
| Risk Level | Low infection risk due to aseptic barrier | Moderate risk; suitable for non-sterile environments |
| Use Case | Surgical instruments, implants | Diagnostic tools, external devices |
| Packaging Material | Sterilizable materials with validated barrier properties | Clean, protective materials without sterile validation |
| Regulatory Compliance | Requires adherence to ISO 11607, FDA sterile device guidelines | Complies with general medical device packaging standards |
| Cost | Higher due to sterilization and validation processes | Lower; less complex process |
| Shelf Life | Extended if integrity maintained | Shorter due to lack of sterile barrier |
Understanding Sterile Packaging in Medical Devices
Sterile packaging in medical devices ensures complete elimination of microbial contamination through validated sterilization processes such as ethylene oxide gas, gamma irradiation, or steam sterilization, maintaining product sterility until use. This packaging type employs barrier materials like Tyvek or laminated films that protect against contaminants and preserve sterility during storage and transportation. Understanding sterile packaging is critical for compliance with regulatory standards such as ISO 11607, which governs packaging systems for terminally sterilized medical products.
What is Clean Packaging?
Clean packaging in medical devices refers to a method that minimizes contamination by using controlled environments and materials that prevent microbial growth, but it does not guarantee sterility like sterile packaging does. This process involves rigorous cleaning and environmental controls to maintain product hygiene while allowing for non-sterile but contaminant-reduced packaging. Clean packaging is ideal for devices that require protection from particulate matter and surface contaminants without the need for terminal sterilization.
Key Differences Between Sterile and Clean Packaging
Sterile packaging ensures medical devices are completely free from viable microorganisms, achieved through rigorous sterilization processes like ethylene oxide or gamma radiation, whereas clean packaging only reduces the microbial load to acceptable levels without full sterilization. Sterile packaging demands stringent validation and monitoring to maintain aseptic conditions, while clean packaging primarily focuses on contamination control through cleanliness standards in the packaging environment. These differences impact regulatory requirements, shelf life, and the critical use cases of medical devices in surgical or invasive procedures.
Regulatory Requirements for Medical Device Packaging
Sterile packaging for medical devices must comply with ISO 11607 standards to ensure aseptic conditions and maintain sterility throughout the product's shelf life, meeting FDA's strict regulatory requirements for implantable and invasive devices. Clean packaging, while reducing microbial contamination, does not guarantee sterility and is regulated under less stringent guidelines such as GMP (Good Manufacturing Practices) for non-sterile devices. Regulatory bodies emphasize validation of sterilization processes, integrity testing, and compliance documentation to ensure patient safety and device efficacy in both packaging types.
Benefits of Sterile Packaging for Patient Safety
Sterile packaging ensures medical devices are free from all viable microorganisms, significantly reducing the risk of infections during surgical procedures and patient care. This form of packaging maintains aseptic conditions until the point of use, enhancing patient safety by preventing contamination-related complications. By meeting stringent regulatory standards, sterile packaging supports reliable infection control protocols essential in healthcare settings.
Applications Suitable for Clean Packaging
Clean packaging is suitable for medical devices that do not require complete sterility but need protection from contamination, such as non-invasive tools and diagnostic equipment. This packaging maintains a controlled environment by using materials and processes that minimize microbial presence without the rigorous sterilization steps. Applications include devices used in clinical settings where aseptic handling is ensured, reducing costs and preserving device integrity.
Materials Used in Sterile vs Clean Packaging
Sterile packaging for medical devices uses materials such as Tyvek, medical-grade films, and foil laminates designed to maintain sterility and resist microbial penetration. Clean packaging employs materials like polyethylene and polypropylene films that ensure cleanliness but do not guarantee sterility. The choice of materials directly impacts the packaging's ability to protect against contamination and preserve device integrity during storage and transport.
Packaging Process: Sterilization vs Cleanroom Assembly
Sterile packaging involves sealing medical devices in a controlled environment followed by terminal sterilization methods such as gamma irradiation or ethylene oxide to ensure elimination of all microbial life. Clean packaging is conducted within ISO-classified cleanrooms where devices are assembled and packaged under stringent contamination control without terminal sterilization. The sterilization in sterile packaging guarantees sterility assurance levels (SAL) of 10^-6, whereas clean packaging relies on rigorous environmental controls and aseptic techniques to maintain cleanliness but does not assure sterility.
Choosing the Right Packaging for Your Medical Device
Sterile packaging ensures a contamination-free environment essential for invasive medical devices, using methods like gamma radiation or ethylene oxide sterilization to maintain microbial safety. Clean packaging provides controlled cleanliness suitable for non-invasive devices or components that do not require sterility but still demand protection from particulate and microbial contamination. Selecting the right packaging depends on the device's intended use, regulatory requirements such as ISO 11607, and the risk assessment of contamination to ensure patient safety and product efficacy.
Impact of Packaging Choice on Compliance and Market Access
Sterile packaging ensures devices remain free from viable microorganisms, meeting stringent regulatory standards essential for market approval and patient safety. Clean packaging, while reducing bioburden, may not satisfy all regulatory bodies' sterility requirements, potentially limiting product access in highly regulated markets. Choosing sterile packaging enhances compliance with global standards such as ISO 13485 and FDA CFR Title 21, facilitating broader market entry and reducing the risk of recalls.
Sterile Packaging vs Clean Packaging Infographic
 productdif.com
productdif.com