Diagnostic medical devices identify and monitor diseases or conditions through tools like imaging systems and blood analyzers, enabling precise detection and early intervention. Therapeutic devices deliver treatment directly to patients, including pacemakers, insulin pumps, and surgical instruments, designed to manage or cure medical conditions. Both types play crucial roles in patient care, with diagnostics guiding the appropriate therapeutic approach to improve health outcomes.
Table of Comparison
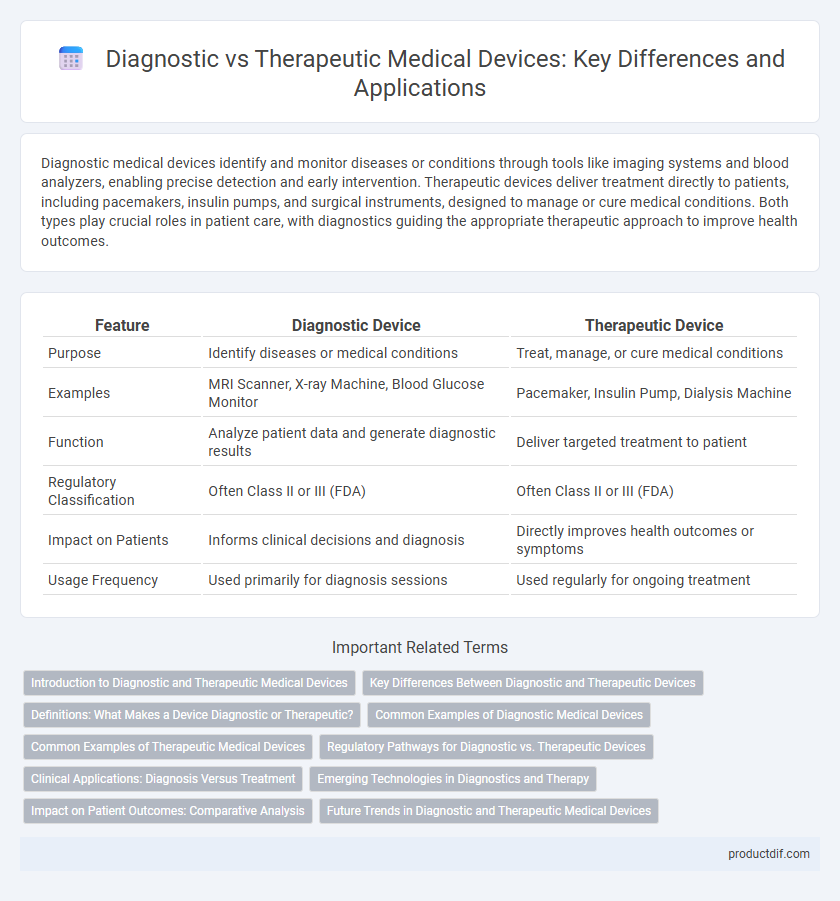
| Feature | Diagnostic Device | Therapeutic Device |
|---|---|---|
| Purpose | Identify diseases or medical conditions | Treat, manage, or cure medical conditions |
| Examples | MRI Scanner, X-ray Machine, Blood Glucose Monitor | Pacemaker, Insulin Pump, Dialysis Machine |
| Function | Analyze patient data and generate diagnostic results | Deliver targeted treatment to patient |
| Regulatory Classification | Often Class II or III (FDA) | Often Class II or III (FDA) |
| Impact on Patients | Informs clinical decisions and diagnosis | Directly improves health outcomes or symptoms |
| Usage Frequency | Used primarily for diagnosis sessions | Used regularly for ongoing treatment |
Introduction to Diagnostic and Therapeutic Medical Devices
Diagnostic medical devices, including imaging systems and laboratory analyzers, are essential for accurately identifying diseases and conditions by detecting physiological markers or abnormalities. Therapeutic medical devices, such as pacemakers, insulin pumps, and surgical instruments, are designed to treat, manage, or alleviate symptoms of medical conditions, improving patient outcomes. Both categories rely on advanced technology to enhance clinical decision-making and patient care throughout diagnosis and treatment processes.
Key Differences Between Diagnostic and Therapeutic Devices
Diagnostic devices are designed to detect, monitor, or diagnose medical conditions by analyzing patient data through imaging, biochemical assays, or physiological measurements. Therapeutic devices provide treatment or intervention to manage, cure, or alleviate medical issues, including surgical instruments, implantable devices, and drug delivery systems. Key differences lie in their primary functions--diagnostic tools identify health problems, while therapeutic devices actively address or modify the condition.
Definitions: What Makes a Device Diagnostic or Therapeutic?
Diagnostic devices are designed to detect, monitor, or diagnose medical conditions by analyzing biological samples or physiological data, such as blood glucose meters and MRI scanners. Therapeutic devices deliver treatment or aid in the management of diseases to improve patient health, including pacemakers and insulin pumps. The fundamental distinction lies in the device's primary purpose: diagnostics identify health issues, while therapeutics actively intervene to treat or alleviate those issues.
Common Examples of Diagnostic Medical Devices
Diagnostic medical devices include imaging equipment such as X-ray machines, MRI scanners, and ultrasound devices that help visualize internal body structures for accurate diagnosis. Laboratory analyzers and blood glucose monitors are widely used to detect diseases and monitor health conditions by analyzing biological samples. Common diagnostic tools also encompass electrocardiograms (ECG) and pulse oximeters, essential for assessing cardiac function and oxygen levels in patients.
Common Examples of Therapeutic Medical Devices
Common examples of therapeutic medical devices include pacemakers, insulin pumps, and dialysis machines, which actively treat or manage medical conditions. These devices work by delivering therapies or interventions directly to patients, improving health outcomes and quality of life. Unlike diagnostic devices that detect or monitor diseases, therapeutic devices play a crucial role in treatment and disease management in clinical settings.
Regulatory Pathways for Diagnostic vs. Therapeutic Devices
Diagnostic devices undergo regulatory pathways emphasizing accuracy, safety, and reliability in detecting diseases or conditions, often requiring thorough clinical validation and compliance with standards like the FDA's 510(k) or De Novo classification. Therapeutic devices face rigorous evaluation for safety and efficacy in treatment applications, necessitating comprehensive clinical trials, adherence to premarket approval (PMA) processes, and post-market surveillance to monitor long-term patient impact. Regulatory authorities, including the FDA and EMA, enforce distinct frameworks for diagnostics and therapeutics to ensure appropriate risk management and performance standards tailored to their intended use.
Clinical Applications: Diagnosis Versus Treatment
Diagnostic medical devices are primarily used to detect and identify diseases through imaging, lab testing, or monitoring, enabling precise clinical decision-making. Therapeutic devices focus on treatment by delivering targeted interventions such as drug delivery systems, implantable devices, or surgical instruments that improve patient outcomes. Clinical applications underscore that diagnostic tools guide treatment strategies, while therapeutic devices actively address medical conditions to restore health.
Emerging Technologies in Diagnostics and Therapy
Emerging technologies in diagnostics leverage advanced imaging, molecular diagnostics, and AI-driven data analysis to enhance early disease detection and personalized treatment planning. Therapeutic innovations incorporate minimally invasive techniques, targeted drug delivery systems, and regenerative medicine approaches to improve patient outcomes and reduce recovery times. Integration of real-time monitoring devices and telemedicine platforms further bridges diagnostics and therapy, enabling continuous patient management and adaptive interventions.
Impact on Patient Outcomes: Comparative Analysis
Diagnostic medical devices enable early detection and accurate identification of diseases, significantly improving patient outcomes by facilitating timely interventions. Therapeutic devices directly treat or manage health conditions, aiming to reduce symptoms and enhance quality of life through targeted interventions. Comparative analysis shows that while diagnostic devices optimize treatment pathways, therapeutic devices deliver measurable improvements in disease progression and patient functionality.
Future Trends in Diagnostic and Therapeutic Medical Devices
Future trends in diagnostic medical devices emphasize integration of artificial intelligence and machine learning to enable early disease detection with higher accuracy and personalized data analysis. Therapeutic medical devices are advancing toward minimally invasive, wearable, and implantable technologies that deliver targeted drug delivery and real-time health monitoring. The convergence of diagnostics and therapeutics through smart, connected devices is revolutionizing patient care by enabling predictive analytics and adaptive treatment protocols.
Diagnostic vs Therapeutic Infographic
 productdif.com
productdif.com