Active implantable medical devices, such as pacemakers and cochlear implants, deliver continuous therapeutic functions by being surgically placed inside the body. Non-implantable medical devices include external monitors and diagnostic tools that operate outside the body, offering less invasive solutions. Understanding the differences in application, risk, and maintenance is crucial for effective patient care and device selection.
Table of Comparison
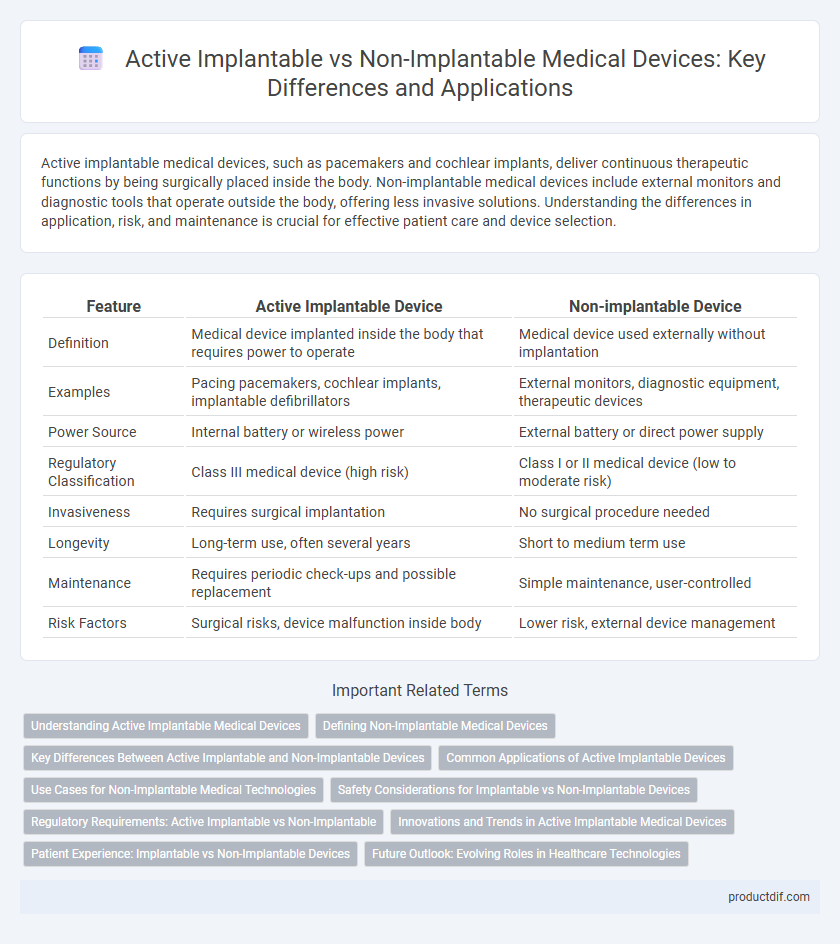
| Feature | Active Implantable Device | Non-implantable Device |
|---|---|---|
| Definition | Medical device implanted inside the body that requires power to operate | Medical device used externally without implantation |
| Examples | Pacing pacemakers, cochlear implants, implantable defibrillators | External monitors, diagnostic equipment, therapeutic devices |
| Power Source | Internal battery or wireless power | External battery or direct power supply |
| Regulatory Classification | Class III medical device (high risk) | Class I or II medical device (low to moderate risk) |
| Invasiveness | Requires surgical implantation | No surgical procedure needed |
| Longevity | Long-term use, often several years | Short to medium term use |
| Maintenance | Requires periodic check-ups and possible replacement | Simple maintenance, user-controlled |
| Risk Factors | Surgical risks, device malfunction inside body | Lower risk, external device management |
Understanding Active Implantable Medical Devices
Active implantable medical devices, such as pacemakers and cochlear implants, are designed to be surgically placed inside the body to provide continuous therapeutic functions. Unlike non-implantable devices, these active implants require power sources like batteries or electromagnetic energy to function autonomously. Understanding the unique regulatory requirements and biocompatibility standards for active implantable devices is crucial for ensuring patient safety and device efficacy.
Defining Non-Implantable Medical Devices
Non-implantable medical devices are external tools or equipment designed for diagnosis, monitoring, or treatment without being surgically placed inside the body. Examples include blood pressure monitors, infusion pumps, and external defibrillators, which provide critical medical functions while minimizing risks associated with invasive procedures. Regulatory agencies classify these devices separately from active implantable devices due to their distinct usage, design, and safety requirements.
Key Differences Between Active Implantable and Non-Implantable Devices
Active implantable medical devices, such as pacemakers and cochlear implants, are surgically placed inside the body and rely on electrical energy to perform their functions continuously or intermittently. In contrast, non-implantable devices, including external hearing aids and blood glucose monitors, remain outside the body and typically provide diagnostic or therapeutic support without internal surgical placement. Key differences include power source dependency, biocompatibility requirements, and regulatory standards related to implantation safety and long-term bodily interaction.
Common Applications of Active Implantable Devices
Active implantable devices include pacemakers, cochlear implants, and neurostimulators, which are designed to be surgically placed inside the body to provide continuous therapeutic benefits. These devices are commonly used to regulate heart rhythms, restore hearing, and manage chronic pain or neurological disorders. Their implantation enables real-time monitoring and intervention, improving patient outcomes in cardiac, auditory, and neurological health.
Use Cases for Non-Implantable Medical Technologies
Non-implantable medical devices encompass a broad range of diagnostic and therapeutic tools used externally, such as infusion pumps, defibrillators, and patient monitoring systems, critical for acute care and chronic disease management. These devices facilitate real-time data collection and intervention without surgical insertion, enhancing patient safety and convenience in outpatient and hospital settings. Non-implantable technologies are essential for applications requiring temporary use or frequent adjustments, including respiratory therapy, wound management, and mobile cardiac monitoring.
Safety Considerations for Implantable vs Non-Implantable Devices
Active implantable medical devices undergo rigorous biocompatibility testing to ensure they do not induce adverse tissue reactions or infections, unlike non-implantable devices which pose less risk due to external placement. Implantable devices demand stringent monitoring for device stability, immune response, and battery longevity to mitigate risks such as device migration or failure. In contrast, non-implantable devices prioritize electrical safety and ergonomic design to prevent external injury or malfunction during patient use.
Regulatory Requirements: Active Implantable vs Non-Implantable
Active implantable medical devices must comply with stringent regulatory requirements such as the European Union's Medical Device Regulation (MDR) 2017/745 and the U.S. FDA's premarket approval (PMA) process due to the risks associated with their placement inside the body. Non-implantable devices, while still regulated under MDR and FDA standards, typically follow a less rigorous pathway like 510(k) clearance or conformity assessment, reflecting their lower risk profile. Both categories require robust clinical evaluation, quality management systems, and post-market surveillance, but active implantables demand enhanced documentation on biocompatibility, electrical safety, and long-term performance.
Innovations and Trends in Active Implantable Medical Devices
Active implantable medical devices, such as pacemakers and neurostimulators, are experiencing rapid innovations in miniaturization, wireless communication, and battery longevity, enhancing patient comfort and device functionality. Advanced sensor integration and AI-driven analytics enable real-time monitoring and personalized therapy adjustments, significantly improving clinical outcomes. Trends in biocompatible materials and smart implants further drive the evolution of active implantable technologies, distinguishing them from non-implantable counterparts by offering continuous, internal therapeutic interventions.
Patient Experience: Implantable vs Non-Implantable Devices
Active implantable medical devices, such as pacemakers and cochlear implants, deliver continuous therapeutic effects directly inside the body, enhancing patient mobility and reducing the need for daily management. In contrast, non-implantable devices like wearable insulin pumps offer flexibility and ease of removal but may require more frequent user intervention and can be less discreet. Patients often report improved quality of life with implantable devices due to their seamless integration and reduced lifestyle disruption, while non-implantable devices provide options for personalized treatment adjustments and easier troubleshooting.
Future Outlook: Evolving Roles in Healthcare Technologies
Active implantable medical devices, such as pacemakers and neurostimulators, are expected to advance through enhanced biocompatibility, miniaturization, and integration with real-time health monitoring systems. Non-implantable devices, including external monitors and portable diagnostic tools, will benefit from improved wireless connectivity and AI-driven predictive analytics to support personalized medicine. The convergence of implantable and non-implantable technologies will reshape patient care by enabling seamless data exchange and proactive health management.
Active Implantable vs Non-implantable Infographic
 productdif.com
productdif.com