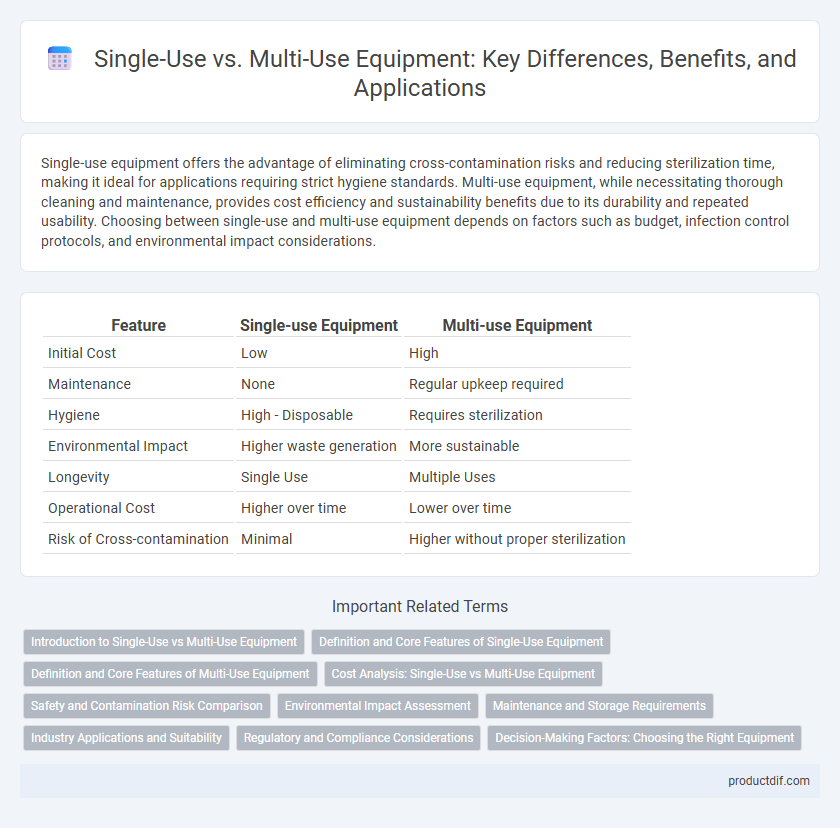
Single-use equipment offers the advantage of eliminating cross-contamination risks and reducing sterilization time, making it ideal for applications requiring strict hygiene standards. Multi-use equipment, while necessitating thorough cleaning and maintenance, provides cost efficiency and sustainability benefits due to its durability and repeated usability. Choosing between single-use and multi-use equipment depends on factors such as budget, infection control protocols, and environmental impact considerations.
Table of Comparison
| Feature | Single-use Equipment | Multi-use Equipment |
|---|---|---|
| Initial Cost | Low | High |
| Maintenance | None | Regular upkeep required |
| Hygiene | High - Disposable | Requires sterilization |
| Environmental Impact | Higher waste generation | More sustainable |
| Longevity | Single Use | Multiple Uses |
| Operational Cost | Higher over time | Lower over time |
| Risk of Cross-contamination | Minimal | Higher without proper sterilization |
Introduction to Single-Use vs Multi-Use Equipment
Single-use equipment is designed for one-time use, offering advantages in sterility and contamination prevention, especially in pharmaceutical and biotech manufacturing. Multi-use equipment, often constructed from stainless steel or durable materials, provides cost efficiency through repeated sterilization and reuse but requires rigorous cleaning validation to avoid cross-contamination. The choice between single-use and multi-use equipment depends on factors like production scale, regulatory requirements, and process flexibility.
Definition and Core Features of Single-Use Equipment
Single-use equipment refers to items designed for one-time use and disposal after a single operation, commonly utilized in medical, pharmaceutical, and laboratory settings to prevent cross-contamination. Core features include sterility, cost-effectiveness due to mass production, and convenience, eliminating the need for cleaning and sterilization processes. These disposable tools enhance safety and efficiency, especially in environments requiring stringent hygiene and contamination control.
Definition and Core Features of Multi-Use Equipment
Multi-use equipment refers to tools and devices designed for repeated use, often constructed from durable materials such as stainless steel or medical-grade plastics to withstand sterilization processes. Core features include high durability, ease of cleaning and sterilization, and compliance with industry standards to ensure safety and functionality over multiple cycles. These characteristics make multi-use equipment essential in settings requiring cost efficiency and environmental sustainability, such as hospitals and laboratories.
Cost Analysis: Single-Use vs Multi-Use Equipment
Single-use equipment typically incurs higher per-unit costs due to frequent replacement, while multi-use equipment requires substantial initial investment but offers lower long-term expenses through repeated use. Maintenance, cleaning, and sterilization costs add to the operational expenses of multi-use equipment, influencing overall cost-effectiveness. Cost analysis should consider factors such as frequency of use, risk of contamination, and lifecycle expenses to determine the optimal choice between single-use and multi-use equipment.
Safety and Contamination Risk Comparison
Single-use equipment significantly reduces contamination risk by eliminating cross-use between patients, ensuring sterility and minimizing infection transmission in medical and laboratory settings. Multi-use equipment requires rigorous cleaning and sterilization protocols to maintain safety, but the potential for human error increases contamination risk. Choosing single-use devices can enhance safety by providing a consistent sterile barrier, while multi-use options demand stringent quality control to prevent safety breaches.
Environmental Impact Assessment
Single-use equipment generates significantly more medical waste, contributing to higher landfill volumes and resource depletion compared to multi-use equipment, which can be sterilized and reused multiple times. The environmental impact assessment reveals that multi-use equipment reduces carbon footprint by lowering the demand for raw materials and energy consumption in manufacturing processes. Lifecycle analyses emphasize that optimizing sterilization techniques and extending the lifespan of multi-use equipment minimizes environmental burdens while maintaining safety and efficacy standards.
Maintenance and Storage Requirements
Single-use equipment requires minimal maintenance and no cleaning, reducing the risk of contamination but increasing disposal costs and environmental impact. Multi-use equipment demands rigorous cleaning protocols, regular inspections, and proper sterilization to ensure safety and functionality, which can increase labor and storage needs. Effective storage solutions for multi-use tools include controlled environments to prevent damage and contamination, whereas single-use items typically require bulk storage space prior to deployment.
Industry Applications and Suitability
Single-use equipment is ideal for biopharmaceutical and sterile manufacturing industries due to its ability to reduce cross-contamination risks and lower cleaning validation requirements. Multi-use equipment suits chemical processing and food production sectors where durability, cost-efficiency, and repetitive usage are critical. Industry-specific factors such as process complexity, batch size, and regulatory compliance dictate the suitability of single-use versus multi-use equipment.
Regulatory and Compliance Considerations
Single-use equipment minimizes contamination risks and simplifies validation processes, aligning with stringent regulatory requirements such as FDA 21 CFR Part 11 and EU GMP Annex 1. Multi-use equipment demands rigorous cleaning and sterilization protocols, comprehensive documentation, and frequent requalification to comply with regulatory standards and prevent cross-contamination. Compliance considerations drive the choice between disposability and reusability, impacting audit readiness and quality control in pharmaceutical and biotech manufacturing.
Decision-Making Factors: Choosing the Right Equipment
Decision-making factors for choosing between single-use and multi-use equipment hinge on cost efficiency, contamination risks, and operational flexibility. Single-use equipment minimizes cleaning requirements and cross-contamination but increases recurring expenses, while multi-use equipment demands stringent sterilization protocols and higher upfront investment. Evaluating production volume, regulatory compliance, and environmental sustainability is essential to optimize equipment selection in manufacturing processes.
Single-use equipment vs multi-use equipment Infographic
 productdif.com
productdif.com