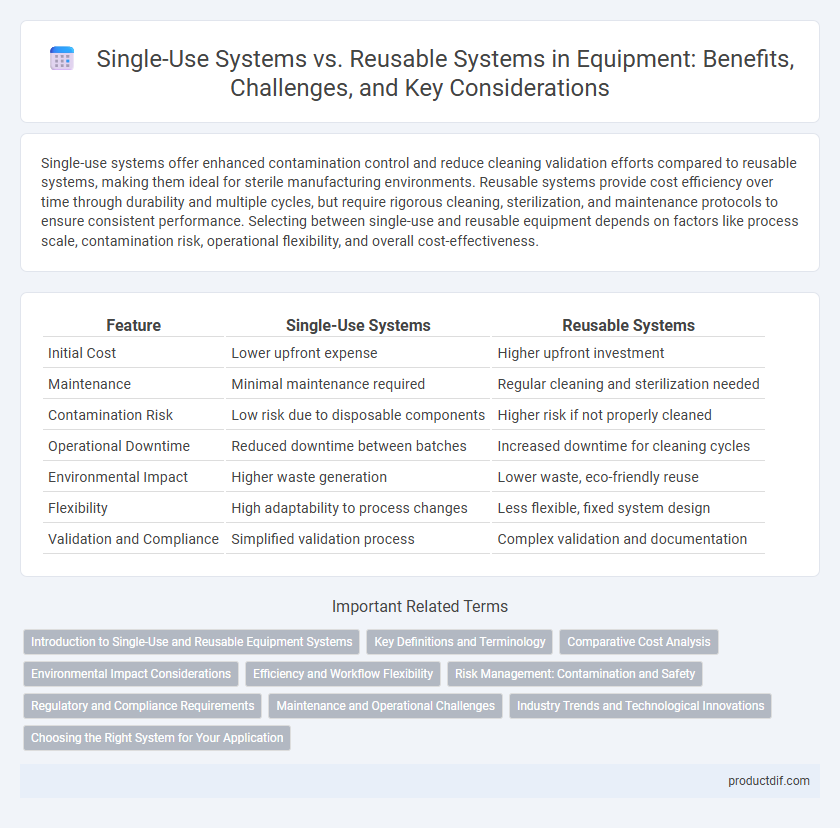
Single-use systems offer enhanced contamination control and reduce cleaning validation efforts compared to reusable systems, making them ideal for sterile manufacturing environments. Reusable systems provide cost efficiency over time through durability and multiple cycles, but require rigorous cleaning, sterilization, and maintenance protocols to ensure consistent performance. Selecting between single-use and reusable equipment depends on factors like process scale, contamination risk, operational flexibility, and overall cost-effectiveness.
Table of Comparison
| Feature | Single-Use Systems | Reusable Systems |
|---|---|---|
| Initial Cost | Lower upfront expense | Higher upfront investment |
| Maintenance | Minimal maintenance required | Regular cleaning and sterilization needed |
| Contamination Risk | Low risk due to disposable components | Higher risk if not properly cleaned |
| Operational Downtime | Reduced downtime between batches | Increased downtime for cleaning cycles |
| Environmental Impact | Higher waste generation | Lower waste, eco-friendly reuse |
| Flexibility | High adaptability to process changes | Less flexible, fixed system design |
| Validation and Compliance | Simplified validation process | Complex validation and documentation |
Introduction to Single-Use and Reusable Equipment Systems
Single-use equipment systems consist of pre-sterilized, disposable components designed for one-time use, significantly reducing the risk of cross-contamination and eliminating cleaning validation processes. Reusable equipment systems, made from durable materials such as stainless steel, require thorough cleaning, sterilization, and maintenance to ensure product quality and regulatory compliance. Choosing between single-use and reusable systems depends on factors like production scale, contamination risk, cost-efficiency, and process flexibility.
Key Definitions and Terminology
Single-use systems (SUS) refer to pre-sterilized, disposable equipment designed for one-time application in bioprocessing, eliminating cleaning requirements and reducing cross-contamination risks. Reusable systems consist of durable materials intended for multiple cycles, requiring validated cleaning and sterilization protocols to ensure consistent performance and regulatory compliance. Understanding terms like sterile barrier, integrity testing, and cleaning validation is essential for distinguishing functional and operational differences between these equipment types.
Comparative Cost Analysis
Single-use systems typically present lower upfront capital expenditures due to reduced infrastructure and sterilization requirements, whereas reusable systems incur higher initial investment but offer cost savings over time through repeated use. Operational expenses for single-use systems often include higher per-batch consumable costs, while reusable systems demand ongoing maintenance, validation, and cleaning resources that can increase labor and utility costs. A comprehensive comparative cost analysis must consider lifecycle costs, including depreciation, downtime, risk of contamination, and regulatory compliance, to determine the most economical choice for specific bioprocessing applications.
Environmental Impact Considerations
Single-use systems generate significant plastic waste, contributing to landfill accumulation and environmental pollution, while reusable systems require energy and water for cleaning and sterilization, impacting carbon footprint. Lifecycle assessments reveal that single-use equipment often has a lower overall environmental impact in water-scarce regions due to reduced cleaning demands. Sustainable practices in equipment selection prioritize minimizing resource consumption and waste generation to balance environmental impacts effectively.
Efficiency and Workflow Flexibility
Single-use systems enhance efficiency by reducing cleaning and sterilization time, allowing for faster changeovers and minimizing downtime in production workflows. Reusable systems offer greater workflow flexibility through adaptability and compatibility with varied processes but require longer preparation and validation times. Balancing the choice between single-use and reusable equipment depends on production scale, process complexity, and the need for rapid turnaround.
Risk Management: Contamination and Safety
Single-use systems significantly reduce contamination risk by eliminating cross-batch contact and minimizing cleaning validation requirements, enhancing overall safety in pharmaceutical and bioprocessing environments. Reusable systems require rigorous cleaning and sterilization protocols to prevent microbial contamination and ensure product safety, increasing operational complexity and risk of human error. Effective risk management mandates thorough validation procedures and monitoring to maintain contamination control in both single-use and reusable equipment setups.
Regulatory and Compliance Requirements
Single-use systems often simplify regulatory compliance by reducing the risk of cross-contamination and eliminating cleaning validation requirements. Reusable systems require rigorous cleaning protocols, detailed documentation, and frequent validation to meet stringent regulatory standards, increasing operational complexity. Compliance agencies emphasize traceability and sterility assurance, making single-use systems advantageous in meeting these regulatory demands efficiently.
Maintenance and Operational Challenges
Single-use systems eliminate the need for cleaning and sterilization, significantly reducing maintenance time and risk of cross-contamination but generate higher waste and recurring costs. Reusable systems require rigorous cleaning, validation, and regular maintenance to ensure operational integrity, increasing labor and downtime demands. Balancing operational challenges involves assessing long-term cost implications, regulatory compliance, and system reliability for production efficiency.
Industry Trends and Technological Innovations
Single-use systems are rapidly gaining traction in biopharmaceutical manufacturing due to their reduced contamination risk, faster setup times, and lower capital investment, aligning with industry trends favoring flexibility and scalability. Technological innovations such as advanced polymer materials and integrated sensor technologies enhance the reliability and monitoring capabilities of single-use equipment, driving efficiency improvements. Conversely, reusable systems continue to evolve with smart cleaning validation protocols and automated sterilization processes, addressing sustainability concerns and operational cost reduction in large-scale production.
Choosing the Right System for Your Application
Selecting between single-use systems and reusable systems depends on factors like process scale, contamination risk, and operational costs. Single-use systems offer flexibility and reduced cleaning requirements, ideal for small batches and rapid changeovers. Reusable systems provide cost efficiency for large-scale production but demand rigorous cleaning and validation protocols to maintain product integrity.
Single-use systems vs Reusable systems Infographic
 productdif.com
productdif.com