The Custom Device Exemption (CDE) allows manufacturers to create personalized medical devices tailored to individual patients without the need for premarket approval, streamlining access for unique clinical needs. In contrast, the Humanitarian Device Exemption (HDE) applies to devices intended to treat or diagnose conditions affecting fewer than 8,000 individuals annually in the U.S., requiring FDA approval based primarily on safety and probable benefit rather than effectiveness. Understanding these regulatory pathways is crucial for developers to ensure timely availability of innovative medical devices while complying with FDA requirements.
Table of Comparison
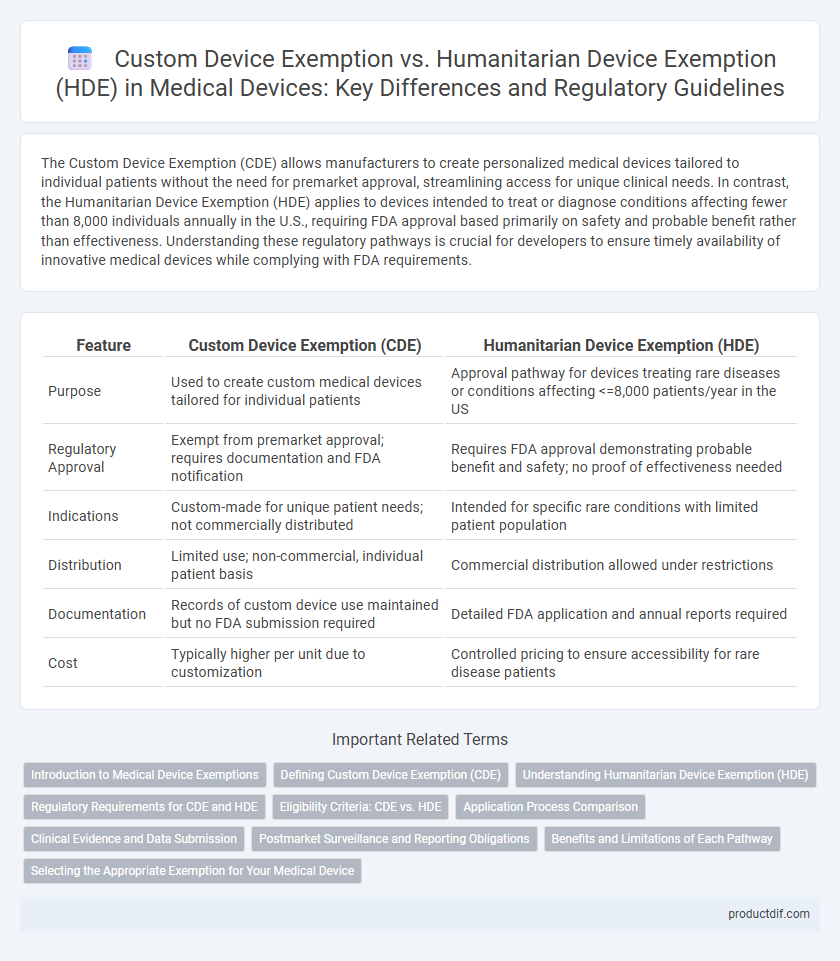
| Feature | Custom Device Exemption (CDE) | Humanitarian Device Exemption (HDE) |
|---|---|---|
| Purpose | Used to create custom medical devices tailored for individual patients | Approval pathway for devices treating rare diseases or conditions affecting <=8,000 patients/year in the US |
| Regulatory Approval | Exempt from premarket approval; requires documentation and FDA notification | Requires FDA approval demonstrating probable benefit and safety; no proof of effectiveness needed |
| Indications | Custom-made for unique patient needs; not commercially distributed | Intended for specific rare conditions with limited patient population |
| Distribution | Limited use; non-commercial, individual patient basis | Commercial distribution allowed under restrictions |
| Documentation | Records of custom device use maintained but no FDA submission required | Detailed FDA application and annual reports required |
| Cost | Typically higher per unit due to customization | Controlled pricing to ensure accessibility for rare disease patients |
Introduction to Medical Device Exemptions
Medical device exemptions streamline regulatory pathways for specific device categories, facilitating faster market access while maintaining safety standards. The Custom Device Exemption (CDE) applies to devices tailored for individual patients, typically unique or rare cases, without the requirement for FDA approval prior to marketing. The Humanitarian Device Exemption (HDE) targets devices intended to benefit patients with rare conditions affecting fewer than 8,000 individuals annually in the United States, allowing marketing approval based on probable benefit rather than proven effectiveness.
Defining Custom Device Exemption (CDE)
The Custom Device Exemption (CDE) allows manufacturers to create medical devices tailored for individual patients or unique clinical situations without requiring premarket approval, provided these devices meet specific criteria under the FDA regulations. Unlike the Humanitarian Device Exemption (HDE), which targets devices intended to treat or diagnose conditions affecting fewer than 8,000 individuals annually in the U.S., the CDE is designed for devices that cannot be generally available in a finished form through labeling or advertising. The CDE ensures access to personalized medical devices while maintaining regulatory oversight to safeguard patient safety and device effectiveness.
Understanding Humanitarian Device Exemption (HDE)
Humanitarian Device Exemption (HDE) permits marketing of medical devices intended to benefit patients with rare conditions affecting fewer than 8,000 individuals annually in the United States. Unlike Custom Device Exemptions, HDE requires FDA approval based on probable benefit without demonstrating effectiveness, facilitating access to innovative devices for small populations. Manufacturers must ensure the device poses no significant risk and complies with post-market surveillance requirements under the HDE pathway.
Regulatory Requirements for CDE and HDE
The Custom Device Exemption (CDE) allows manufacturing of devices tailored for individual patients without requiring premarket approval, provided they meet FDA regulations ensuring device safety and effectiveness. In contrast, the Humanitarian Device Exemption (HDE) applies to devices intended for rare conditions affecting fewer than 8,000 individuals annually, requiring FDA approval based on probable benefit and safety rather than effectiveness. Both exemptions mandate compliance with Quality System Regulations (QSR) and proper labeling, but HDE devices must demonstrate Institutional Review Board (IRB) oversight and post-market surveillance.
Eligibility Criteria: CDE vs. HDE
The Custom Device Exemption (CDE) applies to devices designed for individual patients with rare or unique conditions, lacking available alternatives, while the Humanitarian Device Exemption (HDE) targets devices intended to treat or diagnose diseases affecting fewer than 8,000 individuals annually in the U.S. Eligibility for CDE requires that the device is not generally available and is developed specifically for the patient's unique needs. HDE eligibility demands evidence that the device does not pose an unreasonable risk and offers probable benefit, despite limited effectiveness data due to the small patient population.
Application Process Comparison
The Custom Device Exemption (CDE) application process requires manufacturers to submit detailed documentation justifying the need for a tailored medical device, including patient-specific details and manufacturing specifications, while bypassing extensive premarket approval. In contrast, the Humanitarian Device Exemption (HDE) application mandates demonstrating probable benefit for a condition affecting fewer than 8,000 individuals annually in the U.S., supported by clinical data or literature, but allows for less rigorous efficacy evidence than traditional devices. Both pathways involve FDA review but differ in evidentiary requirements, institutional reporting, and marketing limitations tailored to device use scope and patient population size.
Clinical Evidence and Data Submission
Custom Device Exemption (CDE) allows manufacturers to bypass extensive clinical trials by demonstrating the device is tailored for an individual patient with unique needs, requiring minimal clinical evidence and limited data submission. Humanitarian Device Exemption (HDE) mandates submission of clinical data supporting safety and probable benefit for treating or diagnosing a disease affecting fewer than 8,000 individuals annually, emphasizing evidence even with limited trial populations. Regulatory review for HDE involves detailed scrutiny of clinical outcomes and risk-benefit analysis, whereas CDE prioritizes device customization documentation over large-scale data.
Postmarket Surveillance and Reporting Obligations
Custom Device Exemption (CDE) and Humanitarian Device Exemption (HDE) both have distinct postmarket surveillance requirements reflecting their unique regulatory frameworks. CDE devices typically require less formal postmarket reporting, focusing primarily on adverse event reporting and device modification notifications due to their individualized nature. HDE devices mandate more structured postmarket surveillance, including annual reports to the FDA detailing device usage and any adverse effects, ensuring continued safety and efficacy for the small patient populations they serve.
Benefits and Limitations of Each Pathway
Custom Device Exemption (CDE) allows manufacturers to tailor medical devices to unique patient needs without extensive FDA premarket approval, offering flexibility but limiting broader market distribution and requiring strict adherence to specific patient criteria. Humanitarian Device Exemption (HDE) targets devices for rare diseases or conditions, enabling market access with reduced regulatory burden and without proven effectiveness, though it restricts use to under 8,000 patients per year and limits profit generation. Both pathways provide crucial options for advancing specialized medical device availability while balancing safety, regulatory oversight, and market reach.
Selecting the Appropriate Exemption for Your Medical Device
Selecting the appropriate exemption for your medical device depends on intended use, patient population, and regulatory requirements. Custom Device Exemption (CDE) applies to devices made specifically for an individual patient, tailored to unique anatomical or physiological needs, whereas Humanitarian Device Exemption (HDE) targets devices designed to treat or diagnose rare conditions affecting fewer than 8,000 individuals annually in the United States. Understanding these criteria ensures compliance with FDA guidelines while facilitating access to essential medical technologies.
Custom device exemption vs Humanitarian device exemption (HDE) Infographic
 productdif.com
productdif.com