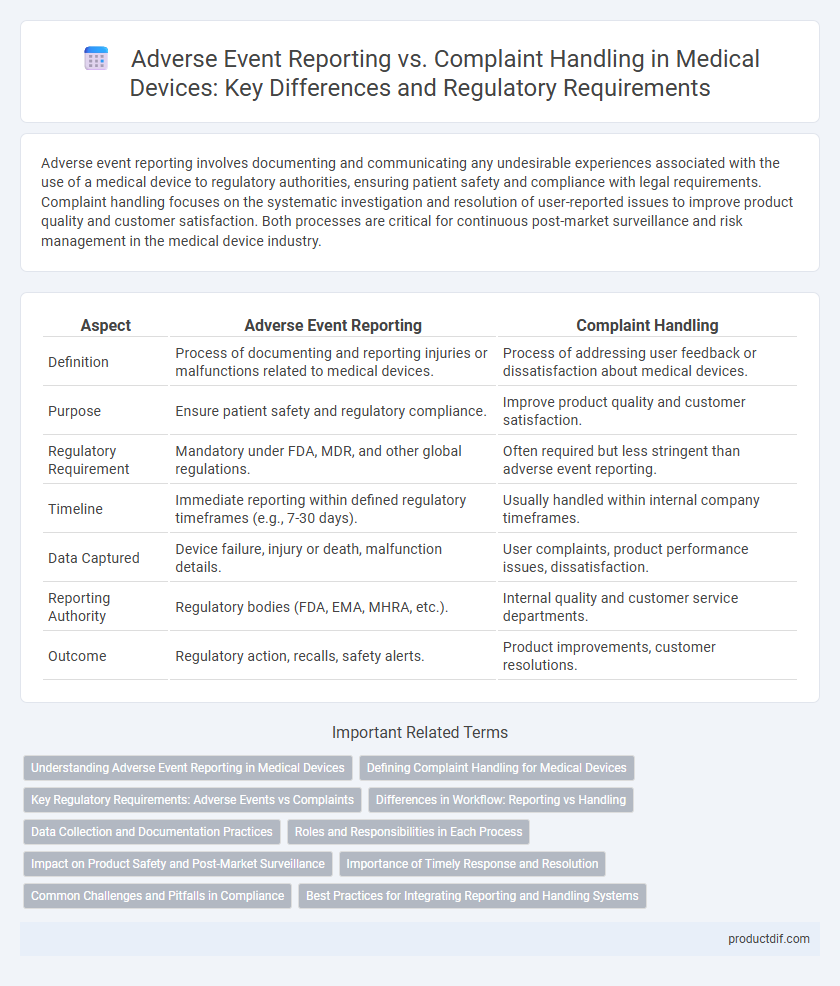
Adverse event reporting involves documenting and communicating any undesirable experiences associated with the use of a medical device to regulatory authorities, ensuring patient safety and compliance with legal requirements. Complaint handling focuses on the systematic investigation and resolution of user-reported issues to improve product quality and customer satisfaction. Both processes are critical for continuous post-market surveillance and risk management in the medical device industry.
Table of Comparison
| Aspect | Adverse Event Reporting | Complaint Handling |
|---|---|---|
| Definition | Process of documenting and reporting injuries or malfunctions related to medical devices. | Process of addressing user feedback or dissatisfaction about medical devices. |
| Purpose | Ensure patient safety and regulatory compliance. | Improve product quality and customer satisfaction. |
| Regulatory Requirement | Mandatory under FDA, MDR, and other global regulations. | Often required but less stringent than adverse event reporting. |
| Timeline | Immediate reporting within defined regulatory timeframes (e.g., 7-30 days). | Usually handled within internal company timeframes. |
| Data Captured | Device failure, injury or death, malfunction details. | User complaints, product performance issues, dissatisfaction. |
| Reporting Authority | Regulatory bodies (FDA, EMA, MHRA, etc.). | Internal quality and customer service departments. |
| Outcome | Regulatory action, recalls, safety alerts. | Product improvements, customer resolutions. |
Understanding Adverse Event Reporting in Medical Devices
Adverse event reporting in medical devices involves the systematic documentation and submission of incidents where a device may have caused or contributed to a serious injury, death, or malfunction, requiring compliance with regulatory authorities like the FDA or EMA. This process ensures timely identification of potential risks and facilitates corrective actions to enhance device safety and efficacy. Unlike complaint handling, which addresses user dissatisfaction and product performance issues, adverse event reporting is a mandatory legal obligation centered on patient protection and post-market surveillance.
Defining Complaint Handling for Medical Devices
Complaint handling for medical devices involves systematically documenting, investigating, and resolving issues reported by users regarding device performance or safety. It ensures compliance with regulatory requirements by identifying potential defects or malfunctions and implementing corrective actions to prevent recurrence. Effective complaint handling supports patient safety, product quality, and continuous improvement in medical device manufacturing.
Key Regulatory Requirements: Adverse Events vs Complaints
Adverse event reporting mandates timely submission of serious incident reports to regulatory authorities, following strict guidelines such as FDA's 21 CFR Part 803 and MDR Article 87. Complaint handling requires manufacturers to document, investigate, and address product complaints per ISO 13485 and 21 CFR Part 820 regulations to ensure ongoing product safety and compliance. Distinguishing adverse events from general complaints is critical for regulatory reporting obligations and risk management in medical device surveillance.
Differences in Workflow: Reporting vs Handling
Adverse event reporting in medical devices involves a structured workflow focused on identifying, documenting, and notifying regulatory authorities about incidents that may compromise patient safety. Complaint handling workflows prioritize capturing and resolving customer feedback or product issues through internal investigations and corrective actions without mandatory regulatory reporting unless a serious adverse event is identified. The key difference lies in the reporting workflow's regulatory compliance emphasis versus the complaint handling process's focus on product improvement and customer satisfaction.
Data Collection and Documentation Practices
Adverse event reporting requires systematic data collection focusing on incidents that result in or may lead to patient harm, with detailed documentation adhering to regulatory standards such as FDA's MedWatch or MDR in the EU. Complaint handling involves gathering user feedback related to device performance, usability, or defects, emphasizing precise recording of product identification, complaint nature, and resolution outcomes to support quality management systems. Effective integration of both processes enhances traceability, risk management, and compliance with ISO 13485 and relevant post-market surveillance requirements.
Roles and Responsibilities in Each Process
Adverse event reporting in medical devices primarily involves regulatory affairs specialists and quality assurance teams tasked with documenting and submitting incident reports to health authorities to ensure compliance with safety regulations. Complaint handling duties fall under customer service and product quality departments, responsible for logging user feedback, investigating product issues, and initiating corrective actions to maintain device performance. Both processes require cross-functional collaboration but differ in their focus: adverse event reporting targets regulatory compliance and patient safety monitoring, while complaint handling emphasizes customer satisfaction and internal quality improvement.
Impact on Product Safety and Post-Market Surveillance
Adverse Event Reporting directly influences product safety by enabling timely identification and mitigation of risks associated with medical devices, ensuring patient protection through regulatory compliance. Complaint Handling serves as a crucial feedback mechanism that detects potential device malfunctions or hazards, supporting continuous improvement and refinement of safety measures. Together, these processes strengthen post-market surveillance by facilitating comprehensive risk assessment and promoting proactive safety management.
Importance of Timely Response and Resolution
Timely response and resolution in adverse event reporting and complaint handling are critical to patient safety and regulatory compliance in the medical device industry. Rapid identification and correction of issues minimize the risk of device malfunction, reduce harm to users, and prevent regulatory penalties from bodies like the FDA or EMA. Efficient handling of complaints and adverse events enhances post-market surveillance and supports continuous device improvement.
Common Challenges and Pitfalls in Compliance
Adverse event reporting and complaint handling in medical devices often face common challenges such as inconsistent data documentation, delayed reporting, and inadequate root cause analysis, which compromise regulatory compliance. Manufacturers frequently struggle with distinguishing between complaints and reportable adverse events, leading to underreporting or misclassification that jeopardizes patient safety and regulatory obligations. Ensuring robust training, clear procedural definitions, and integrated quality management systems is essential to address these pitfalls and maintain compliance with FDA and ISO 13485 standards.
Best Practices for Integrating Reporting and Handling Systems
Integrating adverse event reporting with complaint handling systems enhances regulatory compliance and patient safety by ensuring seamless data flow and timely issue resolution. Best practices include implementing standardized data formats, centralized databases, and automated workflows to capture, assess, and escalate incidents efficiently. Leveraging real-time analytics and cross-functional collaboration improves root cause analysis and preventive action implementation in medical device management.
Adverse Event Reporting vs Complaint Handling Infographic
 productdif.com
productdif.com