Clinical evaluation involves assessing a medical device's safety and performance through trials involving human subjects, providing real-world evidence to support regulatory approval. Preclinical evaluation consists of laboratory and animal testing conducted to understand the device's biological effects and mechanical functionality before human use. Both phases are critical for ensuring the device meets safety standards and performs effectively in intended clinical settings.
Table of Comparison
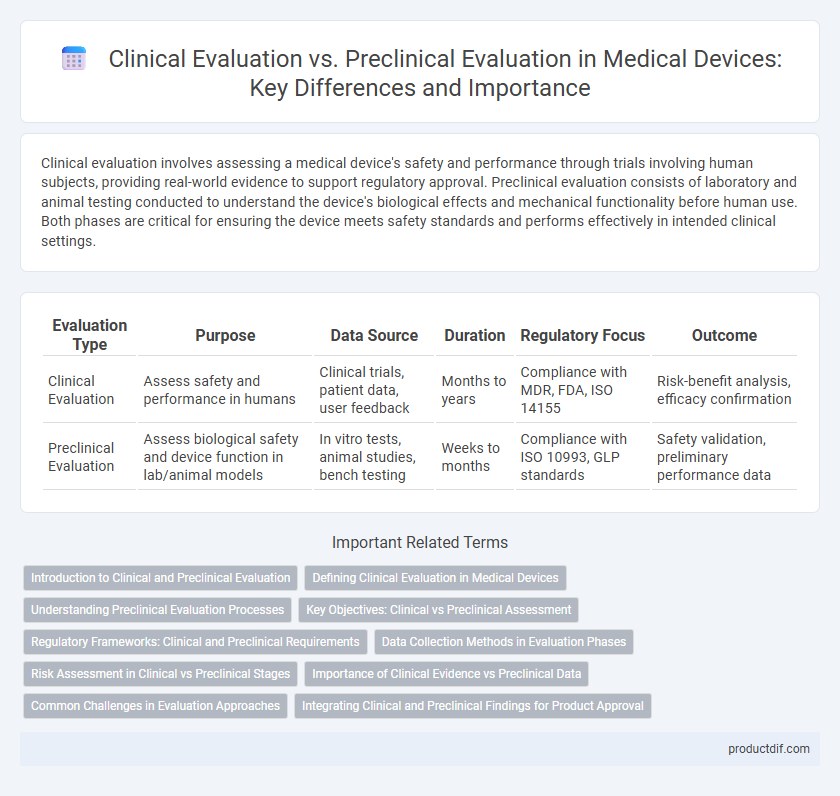
| Evaluation Type | Purpose | Data Source | Duration | Regulatory Focus | Outcome |
|---|---|---|---|---|---|
| Clinical Evaluation | Assess safety and performance in humans | Clinical trials, patient data, user feedback | Months to years | Compliance with MDR, FDA, ISO 14155 | Risk-benefit analysis, efficacy confirmation |
| Preclinical Evaluation | Assess biological safety and device function in lab/animal models | In vitro tests, animal studies, bench testing | Weeks to months | Compliance with ISO 10993, GLP standards | Safety validation, preliminary performance data |
Introduction to Clinical and Preclinical Evaluation
Clinical evaluation assesses a medical device's safety and performance in human subjects through clinical investigations and literature review, providing essential data for regulatory approval. Preclinical evaluation involves laboratory and animal testing to gather initial safety, biocompatibility, and functional data before human trials, forming the foundational evidence for clinical study design. Both evaluations are critical components of the medical device development process, ensuring compliance with regulatory standards like ISO 14155 and MDR.
Defining Clinical Evaluation in Medical Devices
Clinical evaluation in medical devices systematically assesses clinical data to verify device safety and performance under actual conditions of use, complying with regulatory standards such as MDR 2017/745. It involves analyzing clinical investigations, scientific literature, and post-market surveillance data to demonstrate clinical benefit and identify potential risks. This process ensures that the device achieves its intended purpose without compromising patient safety before market approval.
Understanding Preclinical Evaluation Processes
Preclinical evaluation in medical devices involves rigorous laboratory and animal testing to assess safety, biocompatibility, and performance before human trials. This phase ensures identification of potential risks and optimization of design parameters, providing essential data for regulatory submissions. Understanding preclinical evaluation processes is crucial for minimizing clinical trial risks and achieving effective clinical evaluation outcomes.
Key Objectives: Clinical vs Preclinical Assessment
Clinical evaluation focuses on assessing the safety, performance, and clinical benefits of a medical device using data from human trials and real-world use. Preclinical evaluation aims to determine device biocompatibility, mechanical integrity, and functional performance through in vitro tests, bench testing, and animal studies before human exposure. The key objective of clinical assessment is to confirm clinical claims and monitor adverse events, while preclinical assessment ensures initial safety and feasibility for clinical use.
Regulatory Frameworks: Clinical and Preclinical Requirements
Regulatory frameworks for medical devices mandate comprehensive preclinical evaluation involving bench testing, biocompatibility, and animal studies to establish safety and performance prior to human exposure. Clinical evaluation requires systematic collection, analysis, and assessment of clinical data from human subjects to confirm device safety and efficacy under intended use conditions. Both evaluations must comply with standards such as ISO 14155 for clinical investigations and ISO 10993 for biological evaluation, aligning with regulatory bodies like the FDA and MDR for market approval.
Data Collection Methods in Evaluation Phases
Clinical evaluation relies on real-world patient data collected through clinical trials, observational studies, and post-market surveillance to assess the safety and performance of medical devices in human subjects. Preclinical evaluation involves laboratory tests, bench testing, in vitro studies, and animal studies to gather preliminary data on biocompatibility, mechanical properties, and potential biological effects. Data collection methods in the clinical phase emphasize patient outcomes and device interaction in complex physiological environments, while preclinical data focuses on controlled experimental conditions to predict device behavior before human exposure.
Risk Assessment in Clinical vs Preclinical Stages
Risk assessment in clinical evaluation involves analyzing patient-related factors, real-world device performance, and adverse event data to ensure safety and effectiveness under actual use conditions. Preclinical evaluation focuses on identifying potential hazards through in vitro testing, animal studies, and bench tests to predict device behavior before human exposure. Both stages rely on systematic data collection and hazard analysis, but clinical risk assessment integrates direct human response, which is critical for regulatory compliance and post-market surveillance.
Importance of Clinical Evidence vs Preclinical Data
Clinical evidence provides direct insights into a medical device's safety and effectiveness in human subjects, making it crucial for regulatory approval and market acceptance. Preclinical data, derived from laboratory and animal studies, offers essential initial information on biocompatibility, toxicity, and device functionality but cannot fully predict clinical performance. Regulatory bodies prioritize robust clinical evidence to ensure patient safety and device efficacy under real-world conditions.
Common Challenges in Evaluation Approaches
Clinical evaluation and preclinical evaluation of medical devices face common challenges including ensuring data relevance and reliability across diverse biological conditions and patient populations. Both stages require rigorous assessment of safety, performance, and risk management, yet clinical evaluation must navigate ethical constraints and variability in human responses, while preclinical evaluation struggles with predictive accuracy of in vitro and animal models. Balancing regulatory requirements with practical limitations on study design and data extrapolation remains a critical hurdle in establishing comprehensive device efficacy and safety profiles.
Integrating Clinical and Preclinical Findings for Product Approval
Integrating clinical and preclinical findings is crucial for comprehensive medical device approval, ensuring safety and efficacy across diverse use conditions. Preclinical evaluation, including in vitro and in vivo testing, establishes foundational biocompatibility and mechanical performance, while clinical evaluation validates real-world device function and patient outcomes. Regulatory agencies such as the FDA and EMA emphasize harmonizing these data sets to support risk-benefit assessments and facilitate market authorization.
Clinical evaluation vs Preclinical evaluation Infographic
 productdif.com
productdif.com