Software validation in medical devices ensures that programs perform accurately and meet regulatory requirements by verifying functionality, reliability, and safety. Hardware validation focuses on testing physical components to confirm durability, performance under stress, and compliance with safety standards. Both processes are crucial to guarantee overall device efficacy and patient safety in clinical settings.
Table of Comparison
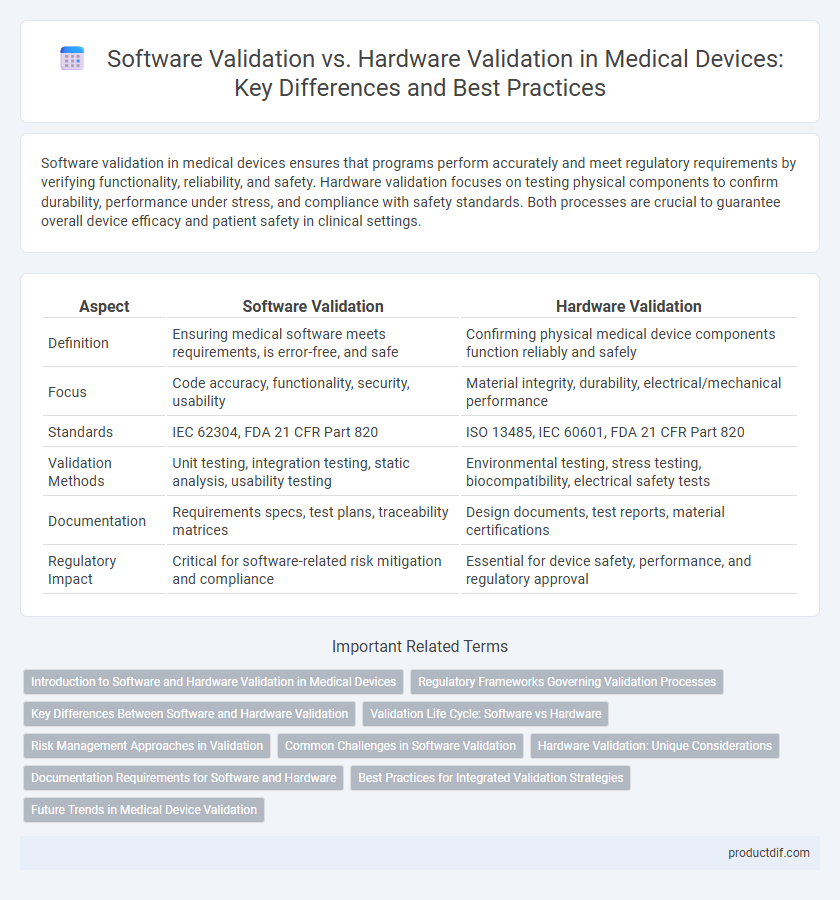
| Aspect | Software Validation | Hardware Validation |
|---|---|---|
| Definition | Ensuring medical software meets requirements, is error-free, and safe | Confirming physical medical device components function reliably and safely |
| Focus | Code accuracy, functionality, security, usability | Material integrity, durability, electrical/mechanical performance |
| Standards | IEC 62304, FDA 21 CFR Part 820 | ISO 13485, IEC 60601, FDA 21 CFR Part 820 |
| Validation Methods | Unit testing, integration testing, static analysis, usability testing | Environmental testing, stress testing, biocompatibility, electrical safety tests |
| Documentation | Requirements specs, test plans, traceability matrices | Design documents, test reports, material certifications |
| Regulatory Impact | Critical for software-related risk mitigation and compliance | Essential for device safety, performance, and regulatory approval |
Introduction to Software and Hardware Validation in Medical Devices
Software validation in medical devices ensures that embedded programs perform correctly and meet regulatory standards like FDA and ISO 13485, focusing on functionality, safety, and data integrity. Hardware validation verifies physical components, including sensors, circuits, and mechanical parts, to guarantee reliability and compliance with performance specifications. Both processes are critical for overall device safety, effectiveness, and regulatory approval in the healthcare industry.
Regulatory Frameworks Governing Validation Processes
Regulatory frameworks such as FDA 21 CFR Part 820 and ISO 13485 establish rigorous validation processes for both software and hardware components in medical devices. Software validation emphasizes compliance with standards like IEC 62304, ensuring thorough risk management and lifecycle documentation. Hardware validation aligns with ISO 14971, focusing on physical device testing, reliability, and safety to meet regulatory requirements.
Key Differences Between Software and Hardware Validation
Software validation in medical devices emphasizes verifying code functionality, logic, and compliance with regulatory standards such as FDA 21 CFR Part 820 and IEC 62304, focusing on risk-based testing and traceability of requirements. Hardware validation centers on physical performance, durability, and safety testing under standards like ISO 13485 and IEC 60601, ensuring device reliability and electrical safety. Key differences include the intangible nature of software requiring static and dynamic testing, while hardware validation involves environmental and mechanical stress assessments to guarantee overall device efficacy and patient safety.
Validation Life Cycle: Software vs Hardware
The validation life cycle for medical devices differs significantly between software and hardware, with software validation emphasizing iterative testing phases such as unit, integration, and system testing to ensure functional correctness and risk mitigation. Hardware validation involves physical testing including environmental, mechanical, and electrical assessments to verify durability, safety, and compliance with regulatory standards. Both processes require thorough documentation and traceability, but software validation often demands continuous updates and regression testing due to evolving code changes, whereas hardware validation focuses on fixed design verification before production.
Risk Management Approaches in Validation
Software validation in medical devices emphasizes dynamic testing and code verification to mitigate risks associated with software failures, ensuring precise functionality and safety under varied clinical scenarios. Hardware validation focuses on physical performance, durability, and compliance with electrical and mechanical safety standards to minimize risks of device malfunction or patient harm. Effective risk management integrates these validation processes by identifying potential failure modes, assessing severity and probability, and implementing controls aligned with ISO 14971 for comprehensive device safety assurance.
Common Challenges in Software Validation
Software validation in medical devices faces common challenges such as ensuring compliance with regulatory standards like FDA 21 CFR Part 820 and ISO 13485, managing complex software updates, and maintaining traceability throughout the development lifecycle. Unlike hardware validation, which emphasizes physical durability and material quality, software validation requires rigorous testing for functionality, security vulnerabilities, and interoperability with other systems. Addressing issues such as ambiguous requirements, software bugs, and configuration management is critical to avoiding costly recalls and ensuring patient safety.
Hardware Validation: Unique Considerations
Hardware validation in medical devices demands rigorous physical testing to ensure durability, safety, and performance under varying environmental conditions such as temperature, humidity, and electromagnetic interference. Unlike software validation, which primarily evaluates code functionality and compliance with regulatory standards like IEC 62304, hardware validation requires compliance with standards such as ISO 13485 and IEC 60601 to verify electrical safety and mechanical integrity. Unique challenges include assessing material biocompatibility, mechanical wear, and failure modes, crucial for patient safety and device reliability.
Documentation Requirements for Software and Hardware
Software validation in medical devices requires comprehensive documentation including software requirements specifications, design documents, code reviews, test plans, and traceability matrices to ensure regulatory compliance and functional accuracy. Hardware validation demands detailed records of design specifications, manufacturing processes, environmental and electrical testing results, and failure mode analysis to confirm device reliability and safety. Both software and hardware validation documentation must align with standards such as ISO 13485 and FDA 21 CFR Part 820 for effective quality management.
Best Practices for Integrated Validation Strategies
Integrated validation strategies in medical devices must encompass both software validation and hardware validation to ensure device safety and regulatory compliance. Software validation focuses on verifying code accuracy, functionality, and cybersecurity measures, while hardware validation emphasizes physical reliability, performance under stress, and electromagnetic compatibility. Employing risk-based approaches, traceability matrices, and continuous verification processes optimizes validation efficiency and supports adherence to standards like FDA 21 CFR Part 820 and ISO 13485.
Future Trends in Medical Device Validation
Future trends in medical device validation emphasize integrating artificial intelligence and machine learning to enhance software validation accuracy and efficiency, enabling predictive analytics for risk management. Hardware validation is evolving with the adoption of advanced sensor technologies and IoT connectivity to ensure real-time performance monitoring and compliance with stringent regulatory standards. Combining software and hardware validation through digital twin technology is becoming a pivotal approach for simulating device behavior and optimizing validation processes in the rapidly advancing medical device industry.
Software Validation vs Hardware Validation Infographic
 productdif.com
productdif.com