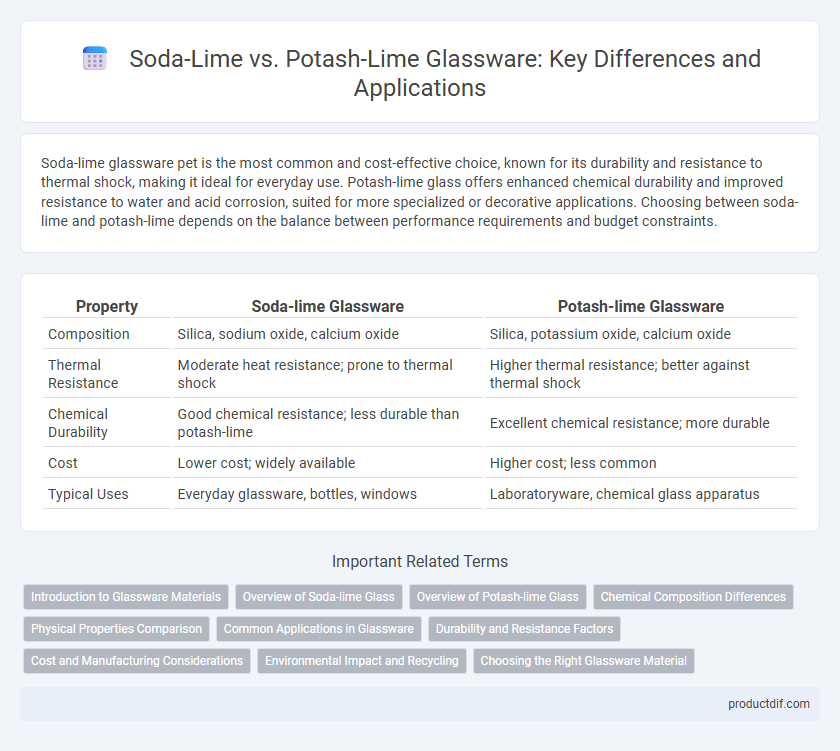
Soda-lime glassware pet is the most common and cost-effective choice, known for its durability and resistance to thermal shock, making it ideal for everyday use. Potash-lime glass offers enhanced chemical durability and improved resistance to water and acid corrosion, suited for more specialized or decorative applications. Choosing between soda-lime and potash-lime depends on the balance between performance requirements and budget constraints.
Table of Comparison
| Property | Soda-lime Glassware | Potash-lime Glassware |
|---|---|---|
| Composition | Silica, sodium oxide, calcium oxide | Silica, potassium oxide, calcium oxide |
| Thermal Resistance | Moderate heat resistance; prone to thermal shock | Higher thermal resistance; better against thermal shock |
| Chemical Durability | Good chemical resistance; less durable than potash-lime | Excellent chemical resistance; more durable |
| Cost | Lower cost; widely available | Higher cost; less common |
| Typical Uses | Everyday glassware, bottles, windows | Laboratoryware, chemical glass apparatus |
Introduction to Glassware Materials
Soda-lime glass, composed primarily of silica, soda (sodium oxide), and lime (calcium oxide), is the most common glass type used in everyday glassware due to its affordability and durability. Potash-lime glass replaces soda with potash (potassium oxide), offering increased chemical resistance and higher melting points, often preferred for specialized applications. Understanding these materials' distinct compositions helps in selecting appropriate glassware based on thermal stability, chemical resistance, and cost considerations.
Overview of Soda-lime Glass
Soda-lime glass is the most common type of glass, composed primarily of silica (SiO2), soda (Na2O), and lime (CaO), which provides durability and ease of melting. It offers excellent chemical stability, thermal resistance, and affordability, making it ideal for windows, bottles, and containers. Compared to potash-lime glass, soda-lime glass has a lower melting point and is more widely used in industrial glass manufacturing.
Overview of Potash-lime Glass
Potash-lime glass is a type of alkali glass primarily composed of potassium oxide (K2O), lime (CaO), and silica (SiO2), known for its superior chemical durability and resistance to water compared to soda-lime glass. It is commonly used in laboratory glassware and specialty containers due to its enhanced thermal and mechanical properties. The higher potassium oxide content results in increased resistance to alkali attack and improved hardness, making potash-lime glass ideal for applications requiring long-term chemical stability.
Chemical Composition Differences
Soda-lime glass primarily consists of silica (SiO2), soda (Na2O), and lime (CaO), with soda acting as a flux to lower melting temperatures. Potash-lime glass differs by using potash (K2O) instead of soda, which enhances chemical durability and resistance to thermal shock. The substitution of potassium oxide for sodium oxide in potash-lime glass results in a denser, more stable glass suited for specific industrial applications.
Physical Properties Comparison
Soda-lime glass, composed primarily of silica, soda (sodium oxide), and lime (calcium oxide), exhibits lower melting points around 1400degC and moderate thermal expansion coefficients, making it versatile for everyday glassware. Potash-lime glass, containing potassium oxide instead of sodium oxide, offers higher melting temperatures near 1450degC and generally improved chemical durability, with reduced thermal expansion leading to enhanced resistance to thermal shock. The denser structure of potash-lime glass contributes to greater hardness and optical clarity, often preferred in high-quality glassware applications.
Common Applications in Glassware
Soda-lime glass is predominantly used in everyday glassware such as bottles, jars, and windows due to its affordability and ease of manufacture. Potash-lime glass, also known as potash glass, is favored for high-quality glassware including laboratory apparatus, fine glass containers, and optical components because of its superior chemical durability and clarity. The choice between soda-lime and potash-lime glass depends on the required strength, chemical resistance, and aesthetic properties for specific glassware applications.
Durability and Resistance Factors
Soda-lime glass, composed mainly of silica, sodium oxide, and calcium oxide, offers moderate durability with good resistance to chemical corrosion but lower resistance to thermal shock compared to potash-lime glass. Potash-lime glass, containing potassium oxide instead of sodium oxide, exhibits enhanced mechanical strength and increased resistance to thermal stress, making it more durable in high-temperature applications. The higher chemical durability and resistance to alkali attack in potash-lime glass contribute to its superior performance in laboratory and industrial glassware.
Cost and Manufacturing Considerations
Soda-lime glass is more cost-effective due to the abundant availability of raw materials like sodium carbonate, making it ideal for mass production. Potash-lime glass, containing potassium carbonate, demands higher manufacturing temperatures and more expensive raw materials, increasing production costs. These factors influence manufacturers' choices between the economic benefits of soda-lime glass and the specific properties of potash-lime glass for specialized applications.
Environmental Impact and Recycling
Soda-lime glass, the most common type of glassware, has a lower environmental impact due to its abundant raw materials and energy-efficient recycling process, producing less CO2 emissions compared to potash-lime glass. Potash-lime glass, traditionally used in specialty glassware, requires more energy-intensive production and presents challenges in recycling due to its higher alkali content, which can complicate batch compatibility and reduce the efficiency of cullet reuse. Efficient recycling of soda-lime glass reduces landfill waste and conserves raw materials, promoting sustainability in the glass manufacturing industry.
Choosing the Right Glassware Material
Soda-lime glass, composed primarily of silica, sodium oxide, and calcium oxide, offers cost-effective durability and is suitable for everyday glassware due to its high thermal resistance and ease of production. Potash-lime glass, containing potassium oxide along with calcium oxide, provides enhanced chemical resistance and a higher refractive index, making it ideal for decorative or laboratory glassware requiring clarity and strength. Selecting the right glassware material depends on the intended use, balancing factors like thermal shock resistance, chemical durability, and optical properties to ensure optimal performance and longevity.
Soda-lime vs Potash-lime Infographic
 productdif.com
productdif.com