Hypochlorite is a powerful disinfectant known for its broad-spectrum antimicrobial activity, making it highly effective in killing bacteria, viruses, and fungi quickly. Quaternary ammonium compounds offer longer-lasting surface protection and are less corrosive, providing safer use on a variety of materials while maintaining antimicrobial efficacy. Choosing between hypochlorite and quaternary ammonium depends on the desired balance between immediate disinfection strength and long-term surface sanitization.
Table of Comparison
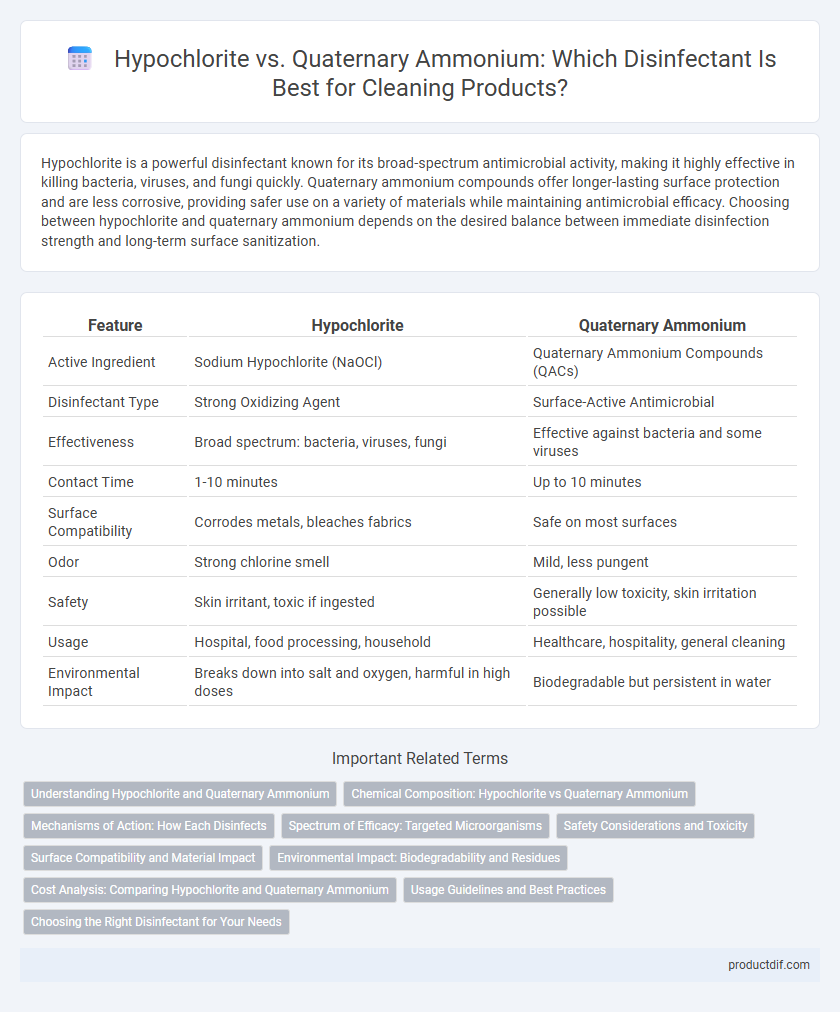
| Feature | Hypochlorite | Quaternary Ammonium |
|---|---|---|
| Active Ingredient | Sodium Hypochlorite (NaOCl) | Quaternary Ammonium Compounds (QACs) |
| Disinfectant Type | Strong Oxidizing Agent | Surface-Active Antimicrobial |
| Effectiveness | Broad spectrum: bacteria, viruses, fungi | Effective against bacteria and some viruses |
| Contact Time | 1-10 minutes | Up to 10 minutes |
| Surface Compatibility | Corrodes metals, bleaches fabrics | Safe on most surfaces |
| Odor | Strong chlorine smell | Mild, less pungent |
| Safety | Skin irritant, toxic if ingested | Generally low toxicity, skin irritation possible |
| Usage | Hospital, food processing, household | Healthcare, hospitality, general cleaning |
| Environmental Impact | Breaks down into salt and oxygen, harmful in high doses | Biodegradable but persistent in water |
Understanding Hypochlorite and Quaternary Ammonium
Hypochlorite is a powerful oxidizing agent widely used for disinfection and bleaching, effective against bacteria, viruses, and fungi by disrupting cellular components. Quaternary ammonium compounds (quats) act as surfactants that disrupt microbial cell membranes, offering broad-spectrum antimicrobial activity with low toxicity and residue. Understanding these agents involves recognizing hypochlorite's strong oxidative mechanism versus quats' membrane-targeting action, which influences their applications and safety profiles in cleaning products.
Chemical Composition: Hypochlorite vs Quaternary Ammonium
Hypochlorite compounds consist of sodium or calcium hypochlorite that release chlorine, providing strong oxidizing properties effective in disinfecting and bleaching. Quaternary ammonium compounds contain nitrogen atoms bonded to four alkyl groups, forming cationic surfactants that disrupt microbial cell membranes. The chemical composition dictates their antimicrobial mechanisms, with hypochlorite relying on chlorine release while quaternary ammonium depends on surface-active properties.
Mechanisms of Action: How Each Disinfects
Hypochlorite disinfects by releasing hypochlorous acid, which penetrates microbial cell walls and oxidizes essential cellular components, leading to rapid microbial death. Quaternary ammonium compounds disrupt cell membranes by interacting with phospholipids, causing leakage of cellular contents and enzyme inactivation. Both agents offer broad-spectrum antimicrobial activity, but hypochlorite acts via strong oxidative damage while quaternary ammoniums compromise membrane integrity.
Spectrum of Efficacy: Targeted Microorganisms
Hypochlorite exhibits a broad-spectrum efficacy, effectively targeting a wide range of bacteria, viruses, fungi, and spores, making it highly versatile for disinfection. Quaternary ammonium compounds (quats) primarily target gram-positive bacteria and some viruses but show limited effectiveness against spores and certain gram-negative bacteria. The choice between hypochlorite and quats depends on the specific microbial threats present in the cleaning environment and desired disinfection outcomes.
Safety Considerations and Toxicity
Hypochlorite, commonly found in bleach, poses risks such as skin and respiratory irritation and can release toxic chlorine gas when mixed with acids, requiring careful ventilation and protective gear during use. Quaternary ammonium compounds (quats) are generally less corrosive but may cause allergic reactions or skin sensitization and have been linked to respiratory issues in sensitive individuals. Proper handling, including wearing gloves and ensuring adequate airflow, is essential to minimize toxicity and maintain safety when using both cleaning agents.
Surface Compatibility and Material Impact
Hypochlorite solutions, such as sodium hypochlorite, are highly effective disinfectants but can cause corrosion and discoloration on metals, plastics, and painted surfaces, limiting their use on delicate materials. Quaternary ammonium compounds (quats) offer broad-spectrum antimicrobial activity with greater surface compatibility, making them safer for use on various non-porous surfaces including vinyl, stainless steel, and sealed wood without damaging finishes. Selecting the appropriate disinfectant depends on the material's resistance to oxidative damage and the cleaning environment's requirements for surface preservation.
Environmental Impact: Biodegradability and Residues
Hypochlorite breaks down into harmless salts and oxygen, making it relatively biodegradable but can release chlorine residues harmful to aquatic life. Quaternary ammonium compounds (quats) have lower biodegradability, tend to persist in the environment, and may accumulate in water sources, posing risks to aquatic organisms. Choosing cleaning products with quaternary ammonium requires careful management of residues to minimize environmental impact.
Cost Analysis: Comparing Hypochlorite and Quaternary Ammonium
Hypochlorite typically offers a lower initial purchase cost compared to quaternary ammonium compounds, making it a budget-friendly choice for large-scale cleaning applications. However, quaternary ammonium compounds often provide longer-lasting antimicrobial effects, potentially reducing overall usage and labor costs over time. Evaluating total cost of ownership including product efficacy, safety measures, and required dilutions ensures a more accurate financial assessment between these disinfectants.
Usage Guidelines and Best Practices
Hypochlorite is commonly used for disinfecting hard, non-porous surfaces and requires dilution according to manufacturer guidelines to avoid surface damage and health hazards. Quaternary ammonium compounds are effective on a broader range of surfaces including plastics and metals, and they remain active longer, making them ideal for routine cleaning in healthcare and food service settings. Proper use involves following contact time recommendations, wearing protective gear, and avoiding mixing these chemicals to prevent toxic reactions.
Choosing the Right Disinfectant for Your Needs
Hypochlorite excels at rapid, broad-spectrum disinfection against bacteria, viruses, and fungi, making it ideal for healthcare and high-risk environments. Quaternary ammonium compounds provide longer-lasting residual antimicrobial activity, suitable for routine surface cleaning in schools, offices, and homes. Selecting the right disinfectant depends on factors like target pathogens, surface compatibility, safety, and contact time requirements.
Hypochlorite vs Quaternary Ammonium Infographic
 productdif.com
productdif.com