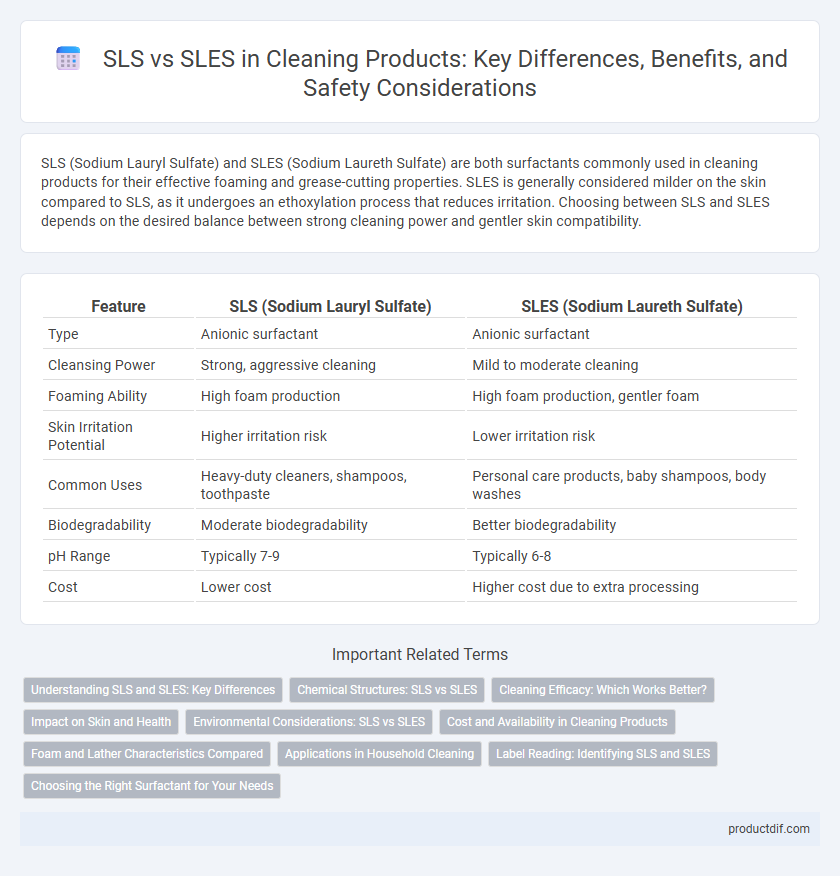
SLS (Sodium Lauryl Sulfate) and SLES (Sodium Laureth Sulfate) are both surfactants commonly used in cleaning products for their effective foaming and grease-cutting properties. SLES is generally considered milder on the skin compared to SLS, as it undergoes an ethoxylation process that reduces irritation. Choosing between SLS and SLES depends on the desired balance between strong cleaning power and gentler skin compatibility.
Table of Comparison
| Feature | SLS (Sodium Lauryl Sulfate) | SLES (Sodium Laureth Sulfate) |
|---|---|---|
| Type | Anionic surfactant | Anionic surfactant |
| Cleansing Power | Strong, aggressive cleaning | Mild to moderate cleaning |
| Foaming Ability | High foam production | High foam production, gentler foam |
| Skin Irritation Potential | Higher irritation risk | Lower irritation risk |
| Common Uses | Heavy-duty cleaners, shampoos, toothpaste | Personal care products, baby shampoos, body washes |
| Biodegradability | Moderate biodegradability | Better biodegradability |
| pH Range | Typically 7-9 | Typically 6-8 |
| Cost | Lower cost | Higher cost due to extra processing |
Understanding SLS and SLES: Key Differences
Sodium Lauryl Sulfate (SLS) and Sodium Laureth Sulfate (SLES) are surfactants widely used in cleaning products for their effective foaming and grease-cutting properties. SLS is known for its strong cleansing power but can be more irritating to sensitive skin, whereas SLES is a milder, ethoxylated derivative designed to reduce skin irritation while maintaining cleaning efficiency. Understanding the chemical structure and skin sensitivity impact of SLS and SLES helps consumers choose appropriate products for their cleaning needs and skin types.
Chemical Structures: SLS vs SLES
Sodium Lauryl Sulfate (SLS) features a sulfate group directly attached to a 12-carbon lauryl chain, making it a strong anionic surfactant known for its effective cleansing and foaming properties. Sodium Laureth Sulfate (SLES) differs by having an ethoxylated chain with one or more ethylene oxide groups inserted between the lauryl chain and the sulfate group, which reduces its irritation potential and improves water solubility. These structural differences influence their behavior in cleaning formulations, with SLES generally considered milder on skin while maintaining efficient detergent action.
Cleaning Efficacy: Which Works Better?
Sodium Lauryl Sulfate (SLS) delivers stronger cleaning power due to its higher foaming ability and aggressive grease-cutting properties, making it ideal for heavy-duty cleaning tasks. Sodium Laureth Sulfate (SLES) offers milder cleansing with less irritation, preferred in products requiring gentler action on skin while maintaining effective dirt removal. For superior cleaning efficacy, SLS outperforms SLES in breaking down oils and grime quickly, but SLES balances performance with skin sensitivity for everyday use.
Impact on Skin and Health
Sodium Lauryl Sulfate (SLS) and Sodium Laureth Sulfate (SLES) are surfactants commonly used in cleaning products, with SLS often causing greater skin irritation and dryness due to its stronger degreasing effects. SLES is a milder alternative, being ethoxylated, which reduces its potential to disrupt the skin barrier and lowers the risk of allergic reactions. Long-term exposure to SLS can lead to increased sensitivity and inflammation, while SLES is generally considered safer for sensitive skin but may still cause irritation in some individuals.
Environmental Considerations: SLS vs SLES
Sodium Lauryl Sulfate (SLS) and Sodium Laureth Sulfate (SLES) both serve as surfactants in cleaning products, but SLS tends to have a higher environmental impact due to its lower biodegradability and potential aquatic toxicity. SLES undergoes ethoxylation, which improves its biodegradability, making it less harmful to aquatic ecosystems compared to SLS. Choosing formulations with SLES over SLS can reduce environmental persistence and contribute to more eco-friendly cleaning solutions.
Cost and Availability in Cleaning Products
Sodium Lauryl Sulfate (SLS) is generally less expensive and more widely available than Sodium Laureth Sulfate (SLES), making it a cost-effective choice for many cleaning products. While SLES offers gentler cleansing properties, manufacturers often select SLS for budget-friendly formulations due to its lower price point and abundant supply. The widespread production and accessibility of SLS contribute to its dominance in low-cost cleaning product markets.
Foam and Lather Characteristics Compared
Sodium Lauryl Sulfate (SLS) produces a rich, dense foam with strong lathering properties that effectively lift and remove dirt and oils from surfaces. Sodium Laureth Sulfate (SLES) generates a smoother, creamier foam that is gentler on the skin while maintaining good cleansing power. SLS tends to create larger bubbles resulting in a more aggressive foam, whereas SLES forms smaller bubbles, offering a milder and more stable lather ideal for sensitive skin formulations.
Applications in Household Cleaning
Sodium Lauryl Sulfate (SLS) and Sodium Laureth Sulfate (SLES) are commonly used surfactants in household cleaning products, with SLES preferred for its milder effect and better skin compatibility. SLS is highly effective in grease removal and foam production, making it suitable for heavy-duty cleaners like degreasers and dishwashing detergents. SLES is often found in surface cleaners, shampoos, and laundry detergents due to its gentler nature and enhanced solubility in water.
Label Reading: Identifying SLS and SLES
Sodium Lauryl Sulfate (SLS) and Sodium Laureth Sulfate (SLES) are common surfactants found on cleaning product labels, often listed under ingredients as detergents or foaming agents. SLS typically appears as "Sodium Lauryl Sulfate" or "Lauryl Sulfate," while SLES may be labeled as "Sodium Laureth Sulfate" or "Laureth Sulfate," indicating an ethoxylated version of SLS. Careful label reading helps consumers identify these compounds to choose products based on skin sensitivity or cleaning power preferences.
Choosing the Right Surfactant for Your Needs
Sodium Lauryl Sulfate (SLS) offers strong cleaning power but can cause skin irritation, making it better suited for heavy-duty cleaning products. Sodium Lauryl Ether Sulfate (SLES) provides a gentler, foam-rich experience, ideal for sensitive skin and household cleaners. Selecting between SLS and SLES depends on balancing cleaning effectiveness with skin sensitivity and environmental impact.
SLS vs SLES Infographic
 productdif.com
productdif.com