Bleach-based cleaners offer powerful disinfecting properties and are highly effective at removing tough stains and killing a wide range of bacteria and viruses. Hydrogen peroxide-based cleaners provide a safer, eco-friendly alternative that breaks down into water and oxygen, making them ideal for sensitive surfaces and indoor air quality. Choosing between the two depends on the cleaning task, surface compatibility, and safety preferences.
Table of Comparison
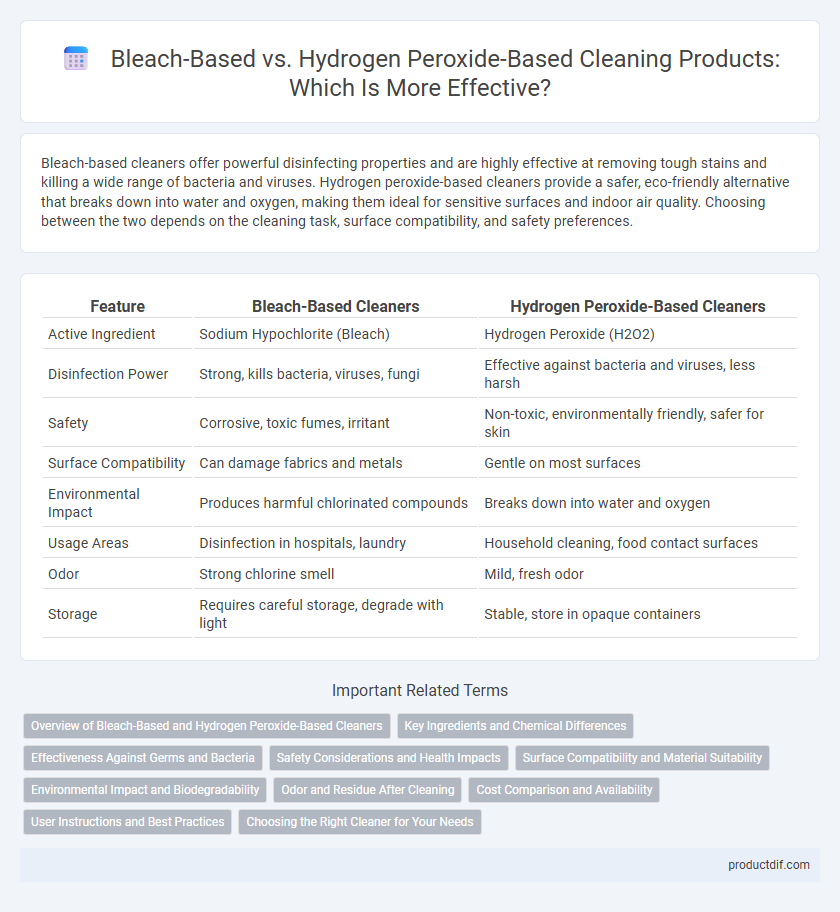
| Feature | Bleach-Based Cleaners | Hydrogen Peroxide-Based Cleaners |
|---|---|---|
| Active Ingredient | Sodium Hypochlorite (Bleach) | Hydrogen Peroxide (H2O2) |
| Disinfection Power | Strong, kills bacteria, viruses, fungi | Effective against bacteria and viruses, less harsh |
| Safety | Corrosive, toxic fumes, irritant | Non-toxic, environmentally friendly, safer for skin |
| Surface Compatibility | Can damage fabrics and metals | Gentle on most surfaces |
| Environmental Impact | Produces harmful chlorinated compounds | Breaks down into water and oxygen |
| Usage Areas | Disinfection in hospitals, laundry | Household cleaning, food contact surfaces |
| Odor | Strong chlorine smell | Mild, fresh odor |
| Storage | Requires careful storage, degrade with light | Stable, store in opaque containers |
Overview of Bleach-Based and Hydrogen Peroxide-Based Cleaners
Bleach-based cleaners contain sodium hypochlorite, providing strong disinfecting power effective against bacteria, viruses, and mold, ideal for heavy-duty cleaning. Hydrogen peroxide-based cleaners offer eco-friendly, non-toxic alternatives that break down into water and oxygen, making them safer for use on various surfaces and in indoor environments. Both types are popular in households and industries, with bleach favored for its potency and hydrogen peroxide valued for its versatility and environmental safety.
Key Ingredients and Chemical Differences
Bleach-based cleaners primarily contain sodium hypochlorite, a powerful oxidizing agent effective in disinfecting and removing tough stains by breaking down organic matter through chlorination. Hydrogen peroxide-based cleaners use H2O2, which releases oxygen radicals that effectively break down stains and kill bacteria without producing harmful chlorinated byproducts. The chemical difference lies in their reactive species: bleach releases chlorine radicals that can be harsh and corrosive, while hydrogen peroxide decomposes into water and oxygen, offering a safer, environmentally friendly alternative for surface cleaning.
Effectiveness Against Germs and Bacteria
Bleach-based cleaners exhibit strong disinfectant properties, effectively eliminating a broad spectrum of germs and bacteria, including tough pathogens like Salmonella and E. coli, due to their powerful oxidizing agents. Hydrogen peroxide-based cleaners offer a safer alternative with comparable antimicrobial efficacy, rapidly breaking down into water and oxygen, reducing harmful residues while targeting viruses and bacteria effectively. Their oxidation process disrupts microbial cell walls, making both options highly efficient, though bleach remains the more potent choice for heavy-duty germ control in high-risk environments.
Safety Considerations and Health Impacts
Bleach-based cleaners release chlorine gas, which can cause respiratory irritation, skin burns, and eye damage, making proper ventilation and protective gear essential during use. Hydrogen peroxide-based cleaners break down into water and oxygen, presenting lower toxicity and reduced risk of harmful fumes, though concentrated forms may still cause skin irritation. Choosing hydrogen peroxide offers safer indoor air quality and less environmental impact, while bleach requires cautious handling to avoid chemical exposure.
Surface Compatibility and Material Suitability
Bleach-based cleaners excel at disinfecting non-porous surfaces like tiled floors and glass but can cause discoloration or corrosion on metals and delicate fabrics. Hydrogen peroxide-based products offer a gentler alternative, effective on a wider range of materials including painted surfaces and textiles without the risk of harsh bleaching or surface degradation. Selecting the appropriate agent depends on surface porosity and material sensitivity to oxidation and harsh chemicals.
Environmental Impact and Biodegradability
Bleach-based cleaners release chlorine compounds that can form harmful byproducts, posing risks to aquatic ecosystems and contributing to water pollution. Hydrogen peroxide-based products break down into water and oxygen, offering a more environmentally friendly and biodegradable alternative with minimal toxic residues. Choosing hydrogen peroxide enhances sustainability and reduces long-term ecological harm compared to traditional bleach formulations.
Odor and Residue After Cleaning
Bleach-based cleaners often leave a strong, lingering chlorine odor that some users find harsh and unpleasant, while hydrogen peroxide-based products tend to dissipate quickly without a noticeable scent. Residue from bleach can sometimes appear as a white, chalky film, requiring additional rinsing to avoid surface damage or discoloration. Hydrogen peroxide usually breaks down into water and oxygen, leaving minimal to no residue after cleaning, making it ideal for sensitive surfaces and odor-sensitive environments.
Cost Comparison and Availability
Bleach-based cleaners generally offer lower upfront costs and are widely available in most retail stores, making them a budget-friendly choice for many households. Hydrogen peroxide-based cleaners tend to be slightly more expensive but are increasingly found in eco-friendly and specialty stores due to their safer environmental profile. While bleach products dominate in affordability and accessibility, hydrogen peroxide options provide good value for consumers prioritizing non-toxic and biodegradable solutions.
User Instructions and Best Practices
Bleach-based cleaners require careful dilution, the use of gloves, and thorough ventilation to prevent respiratory irritation and skin burns during application. Hydrogen peroxide-based products are typically safer for use on a variety of surfaces but should be stored away from light and heat to maintain effectiveness. For best results, apply bleach-based solutions to heavily soiled areas with a contact time of 5-10 minutes, while hydrogen peroxide works optimally when left to sit for at least 3 minutes before wiping.
Choosing the Right Cleaner for Your Needs
Bleach-based cleaners excel in disinfecting and whitening due to their strong antimicrobial properties, making them ideal for heavy-duty sanitation in kitchens and bathrooms. Hydrogen peroxide-based cleaners offer effective stain removal and deodorizing with less toxicity, suitable for sensitive surfaces and eco-friendly households. Selecting the right cleaner depends on surface type, desired disinfection level, and environmental impact considerations.
Bleach-based vs Hydrogen peroxide-based Infographic
 productdif.com
productdif.com