Electroforming and electroplating are two distinct techniques used in jewelry making to add metal coatings, with electroforming involving the buildup of thick metal layers to create solid, lightweight pieces, while electroplating deposits a thin metal layer onto a substrate for a decorative finish. Electroforming offers greater durability and design flexibility, making it ideal for intricate pet-themed jewelry that requires strength and detail. Electroplating provides a cost-effective, aesthetically pleasing surface enhancement but with less durability compared to electroforming.
Table of Comparison
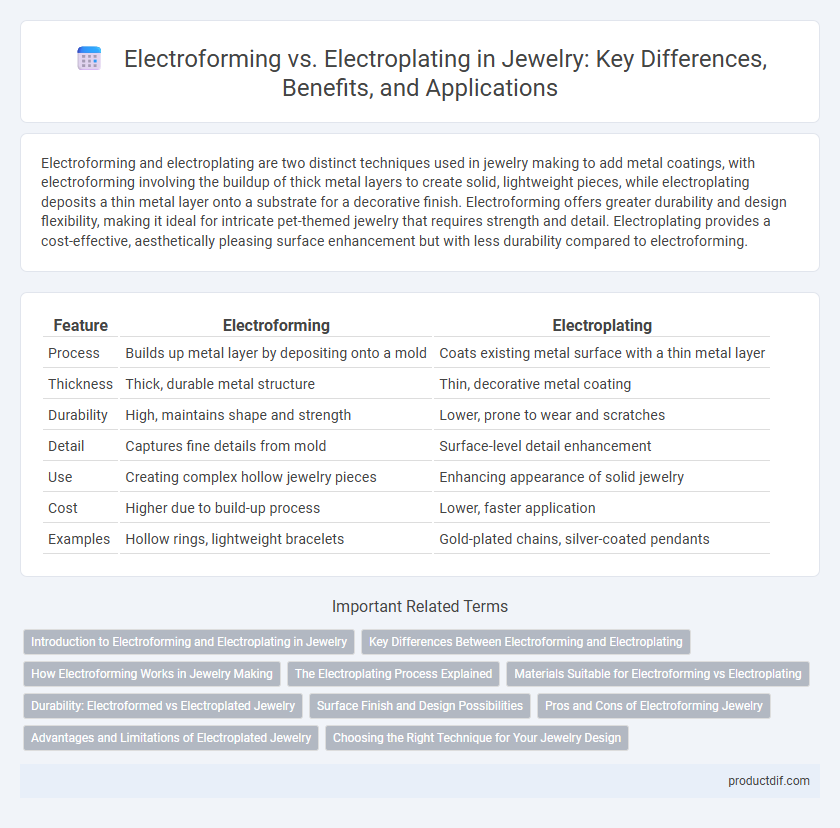
| Feature | Electroforming | Electroplating |
|---|---|---|
| Process | Builds up metal layer by depositing onto a mold | Coats existing metal surface with a thin metal layer |
| Thickness | Thick, durable metal structure | Thin, decorative metal coating |
| Durability | High, maintains shape and strength | Lower, prone to wear and scratches |
| Detail | Captures fine details from mold | Surface-level detail enhancement |
| Use | Creating complex hollow jewelry pieces | Enhancing appearance of solid jewelry |
| Cost | Higher due to build-up process | Lower, faster application |
| Examples | Hollow rings, lightweight bracelets | Gold-plated chains, silver-coated pendants |
Introduction to Electroforming and Electroplating in Jewelry
Electroforming in jewelry involves depositing thick metal layers onto a model, creating lightweight yet durable hollow pieces with intricate details, often using copper or silver. Electroplating applies a thin metal coating over a base metal item to enhance appearance and prevent tarnishing, commonly using gold, silver, or rhodium. Both techniques use electrical currents to reduce metal ions onto surfaces but differ in thickness, durability, and structural applications.
Key Differences Between Electroforming and Electroplating
Electroforming builds up thick, durable metal layers by depositing metal onto a conductive form, enabling the creation of free-standing, hollow jewelry pieces, whereas electroplating applies a thin metal coating primarily for decorative or protective purposes. Electroforming allows for complex, three-dimensional designs by using molds that are later removed, while electroplating enhances existing solid objects without altering their shape. The thickness of metal in electroforming typically ranges from micrometers to millimeters, compared to the usually nanometer-thick layers in electroplating.
How Electroforming Works in Jewelry Making
Electroforming in jewelry making involves depositing a thick layer of metal onto a conductive surface by submerging the object into an electrolyte solution and passing an electric current through it. This process builds up metal gradually, allowing for intricate and hollow designs with precise detail and lightweight structure. Unlike electroplating, which applies a thin metal coating, electroforming creates a durable, standalone metal form ideal for custom and artistic jewelry pieces.
The Electroplating Process Explained
The electroplating process involves depositing a thin layer of metal, such as gold or silver, onto the surface of a jewelry base metal by using an electric current in a metal salt solution. This technique enhances the jewelry's appearance, conductivity, and corrosion resistance while maintaining intricate design details and a smooth finish. Electroplating is commonly used in fine jewelry to create durable, lustrous coatings that protect underlying materials and improve wear resistance.
Materials Suitable for Electroforming vs Electroplating
Electroforming is ideal for materials such as copper, nickel, and silver due to their excellent conductivity and malleability, allowing for the creation of intricate, lightweight jewelry pieces with substantial metal thickness. Electroplating suits a wider range of base materials, including non-conductive substrates like plastic or glass when pre-treated, enabling the deposition of thin metallic layers such as gold, silver, or rhodium for enhanced surface appearance and durability. Choosing between electroforming and electroplating depends on the desired final object's structural properties and the substrate's ability to withstand the electrochemical process.
Durability: Electroformed vs Electroplated Jewelry
Electroformed jewelry boasts superior durability due to its thick, solid metal layer formed through an extended plating process, providing greater resistance to wear and damage compared to electroplated pieces. Electroplated jewelry features a thin metal coating over a base metal, making it more prone to scratching, fading, and peeling over time. Choosing electroformed jewelry ensures longer-lasting shine and strength, ideal for pieces exposed to frequent handling or daily wear.
Surface Finish and Design Possibilities
Electroforming creates thicker, more sculptural metal layers, enabling intricate, hollow designs with durable, textured surface finishes that maintain fine detail. In contrast, electroplating applies a thin, uniform metal coating over existing jewelry, enhancing surface shine and corrosion resistance but limiting structural changes. Designers seeking bold, three-dimensional forms favor electroforming, while electroplating suits enhancing appearance and adding protective layers to finished pieces.
Pros and Cons of Electroforming Jewelry
Electroforming jewelry offers the advantage of creating lightweight, hollow pieces with fine details and greater design freedom compared to traditional methods. It provides excellent durability and thickness, making the pieces more resistant to wear and corrosion, but may require longer production times and higher initial setup costs. However, electroforming can be less suitable for mass production due to slower processing and potential limitations in achieving ultra-thin coatings.
Advantages and Limitations of Electroplated Jewelry
Electroplated jewelry offers a cost-effective way to achieve a high-quality metal finish, enhancing aesthetic appeal with a smooth, shiny surface while allowing for a variety of metal coatings such as gold, silver, and rhodium. The limitations include reduced durability compared to solid or electroformed pieces, as the thin metal layer can wear off or tarnish over time, especially with frequent exposure to moisture, chemicals, or abrasion. Maintenance is required to preserve the plating's appearance, and electroplating does not add significant structural strength to the jewelry item.
Choosing the Right Technique for Your Jewelry Design
Electroforming creates thicker, more durable metal layers ideal for intricate, sculptural jewelry designs, while electroplating offers a thinner, decorative coating suited for enhancing existing pieces with precious metals like gold or silver. Consider the desired durability, weight, and visual effect when choosing between these techniques, as electroforming allows for lightweight, hollow structures, whereas electroplating emphasizes surface finish and color. Selecting the right method enhances both the aesthetic appeal and functional longevity of your jewelry.
Electroforming vs Electroplating Infographic
 productdif.com
productdif.com