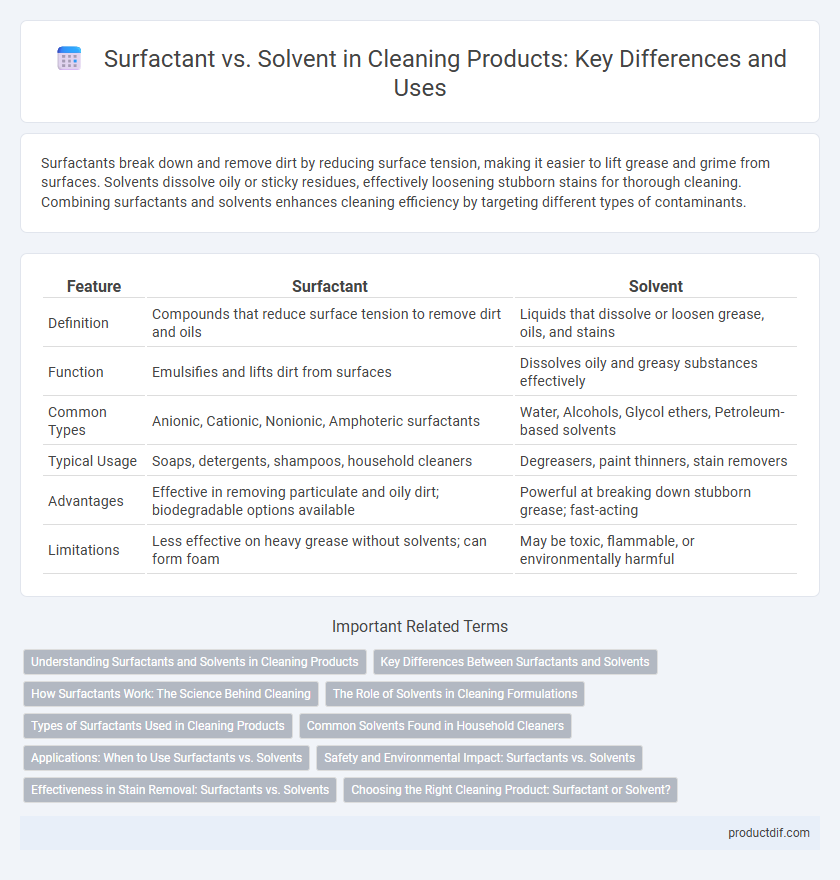
Surfactants break down and remove dirt by reducing surface tension, making it easier to lift grease and grime from surfaces. Solvents dissolve oily or sticky residues, effectively loosening stubborn stains for thorough cleaning. Combining surfactants and solvents enhances cleaning efficiency by targeting different types of contaminants.
Table of Comparison
| Feature | Surfactant | Solvent |
|---|---|---|
| Definition | Compounds that reduce surface tension to remove dirt and oils | Liquids that dissolve or loosen grease, oils, and stains |
| Function | Emulsifies and lifts dirt from surfaces | Dissolves oily and greasy substances effectively |
| Common Types | Anionic, Cationic, Nonionic, Amphoteric surfactants | Water, Alcohols, Glycol ethers, Petroleum-based solvents |
| Typical Usage | Soaps, detergents, shampoos, household cleaners | Degreasers, paint thinners, stain removers |
| Advantages | Effective in removing particulate and oily dirt; biodegradable options available | Powerful at breaking down stubborn grease; fast-acting |
| Limitations | Less effective on heavy grease without solvents; can form foam | May be toxic, flammable, or environmentally harmful |
Understanding Surfactants and Solvents in Cleaning Products
Surfactants reduce surface tension between water and dirt, enabling effective removal of grease and oils by emulsifying contaminants for easy rinsing. Solvents dissolve stubborn substances, such as waxes and resins, breaking down particles to enhance cleaning efficiency in formulations. Understanding the distinct roles of surfactants and solvents helps optimize cleaning product performance for diverse applications.
Key Differences Between Surfactants and Solvents
Surfactants reduce surface tension between liquids and solids, enhancing the emulsification and removal of dirt, while solvents dissolve oily and greasy residues by breaking down chemical bonds. Surfactants contain hydrophilic and hydrophobic groups, enabling them to surround and lift particles from surfaces, whereas solvents primarily act as dissolving agents without altering surface tension. The distinct molecular functions of surfactants and solvents make them essential yet different components in cleaning formulations for effective stain and grime removal.
How Surfactants Work: The Science Behind Cleaning
Surfactants function by reducing the surface tension of water, allowing it to more effectively penetrate and lift dirt, grease, and oils from surfaces. Their molecular structure contains hydrophobic tails that bind to oily substances and hydrophilic heads that attract water, enabling the emulsification and removal of grime. This mechanism differentiates surfactants from solvents, which dissolve contaminants chemically rather than mechanically lifting them from surfaces.
The Role of Solvents in Cleaning Formulations
Solvents in cleaning formulations play a crucial role by dissolving soils, oils, and grease, allowing for effective removal from surfaces. They enhance the overall cleaning power by penetrating and breaking down stubborn residues while supporting the action of surfactants. Common solvents such as water, alcohols, and glycol ethers optimize the efficiency and performance of cleaning products in various applications.
Types of Surfactants Used in Cleaning Products
Surfactants in cleaning products are primarily categorized into anionic, cationic, nonionic, and amphoteric types, each offering distinct properties for stain removal and emulsification. Anionic surfactants like sodium lauryl sulfate excel in heavy-duty cleaning by breaking down grease and dirt, while nonionic surfactants such as alkyl polyglucosides provide gentle yet effective cleaning suitable for sensitive surfaces and fabrics. Cationic surfactants serve as antimicrobial agents and fabric softeners, whereas amphoteric surfactants, including betaines, maintain mildness and foaming in diverse cleaning formulations.
Common Solvents Found in Household Cleaners
Common solvents found in household cleaners include water, alcohols such as isopropanol and ethanol, glycol ethers, and petroleum-based solvents like mineral spirits. These solvents help dissolve oils, greases, and dirt, enhancing the cleaning efficiency of products. Surfactants, in contrast, reduce surface tension and emulsify contaminants, but solvents specifically target chemical dissolution and residue removal.
Applications: When to Use Surfactants vs. Solvents
Surfactants are ideal for removing dirt and grease from surfaces in household and industrial cleaning by lowering surface tension and emulsifying oils. Solvents excel at dissolving stubborn adhesives, inks, and heavy oily residues where surfactants alone may not suffice. Use surfactants for water-based cleaning tasks and solvents for targeting non-polar, tough contaminants requiring strong dissolving properties.
Safety and Environmental Impact: Surfactants vs. Solvents
Surfactants generally present lower toxicity and biodegrade more readily in the environment compared to many traditional solvents, reducing the risk of water and soil contamination. Solvents often contain volatile organic compounds (VOCs) that contribute to air pollution and pose inhalation hazards, impacting both human health and ecosystems. Choosing surfactant-based cleaning products can enhance safety profiles and minimize environmental footprints by leveraging biodegradable ingredients with reduced chemical emissions.
Effectiveness in Stain Removal: Surfactants vs. Solvents
Surfactants excel in stain removal by breaking down and emulsifying oils and grease, allowing water to lift away dirt effectively. Solvents, on the other hand, dissolve stubborn stains such as ink, paint, and adhesives by chemically breaking down their molecular structure. Combining surfactants and solvents in cleaning products maximizes stain removal by targeting a broader range of contaminants with complementary mechanisms.
Choosing the Right Cleaning Product: Surfactant or Solvent?
Surfactants reduce surface tension between water and dirt, making them ideal for removing grease and oils from surfaces in household and industrial cleaning. Solvents dissolve stubborn stains and residues by breaking down chemical bonds, often used for paint removal and heavy-duty degreasing. Selecting the right cleaning product depends on the type of soil and surface, with surfactants preferred for general cleaning and solvents for targeted stain removal.
Surfactant vs Solvent Infographic
 productdif.com
productdif.com