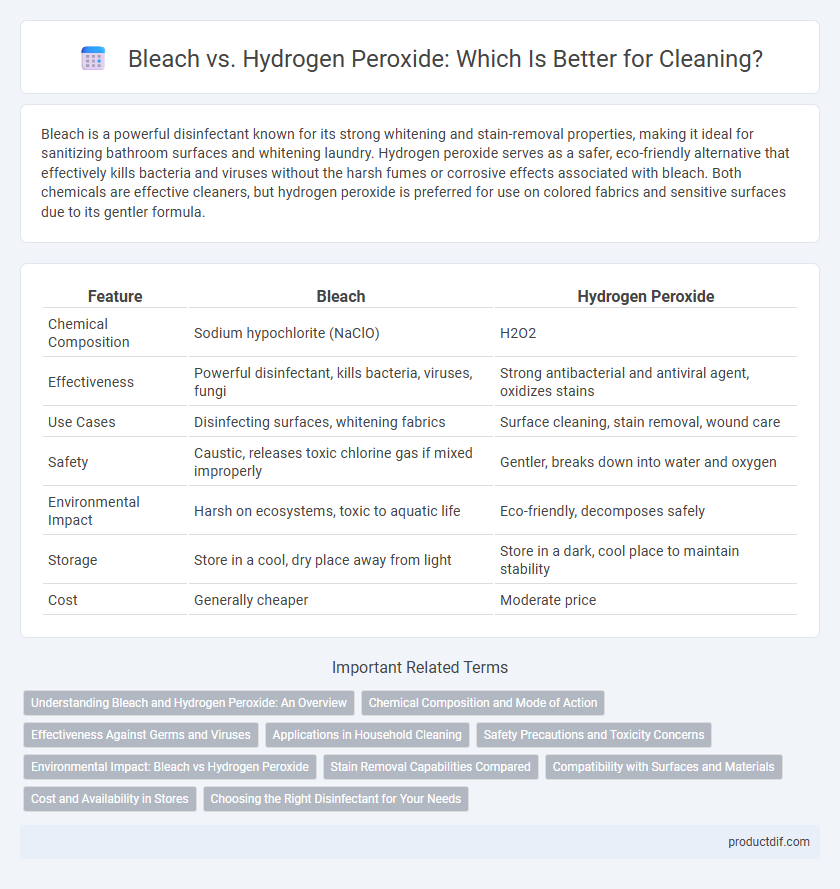
Bleach is a powerful disinfectant known for its strong whitening and stain-removal properties, making it ideal for sanitizing bathroom surfaces and whitening laundry. Hydrogen peroxide serves as a safer, eco-friendly alternative that effectively kills bacteria and viruses without the harsh fumes or corrosive effects associated with bleach. Both chemicals are effective cleaners, but hydrogen peroxide is preferred for use on colored fabrics and sensitive surfaces due to its gentler formula.
Table of Comparison
| Feature | Bleach | Hydrogen Peroxide |
|---|---|---|
| Chemical Composition | Sodium hypochlorite (NaClO) | H2O2 |
| Effectiveness | Powerful disinfectant, kills bacteria, viruses, fungi | Strong antibacterial and antiviral agent, oxidizes stains |
| Use Cases | Disinfecting surfaces, whitening fabrics | Surface cleaning, stain removal, wound care |
| Safety | Caustic, releases toxic chlorine gas if mixed improperly | Gentler, breaks down into water and oxygen |
| Environmental Impact | Harsh on ecosystems, toxic to aquatic life | Eco-friendly, decomposes safely |
| Storage | Store in a cool, dry place away from light | Store in a dark, cool place to maintain stability |
| Cost | Generally cheaper | Moderate price |
Understanding Bleach and Hydrogen Peroxide: An Overview
Bleach, primarily composed of sodium hypochlorite, acts as a powerful disinfectant and stain remover by breaking down molecules through oxidation. Hydrogen peroxide serves as a milder oxidizing agent effective for sanitizing and whitening without producing harmful byproducts, decomposing into water and oxygen. Both substances excel in cleaning but differ in their chemical properties, safety profiles, and suitable applications for household or industrial use.
Chemical Composition and Mode of Action
Bleach, primarily composed of sodium hypochlorite, acts as a strong oxidizing agent that breaks down organic stains and disinfects surfaces by releasing chlorine. Hydrogen peroxide, containing H2O2 molecules, decomposes into water and oxygen, producing reactive oxygen species that disrupt cellular components of microbes and help remove stains. Both chemicals are effective disinfectants but differ in corrosiveness and environmental impact due to their distinct chemical compositions and reaction mechanisms.
Effectiveness Against Germs and Viruses
Bleach is highly effective against a broad spectrum of germs and viruses, including tough pathogens like norovirus and influenza, due to its strong oxidizing properties. Hydrogen peroxide also disinfects efficiently by producing free radicals that destroy microbial cells, making it effective against bacteria, viruses, and spores, though typically at a slower rate than bleach. Both agents are widely used for sanitation, but bleach remains the preferred choice in healthcare settings for rapid, high-level germicidal activity.
Applications in Household Cleaning
Bleach is highly effective for disinfecting surfaces, whitening fabrics, and removing mold due to its strong oxidizing properties and broad-spectrum antimicrobial action. Hydrogen peroxide excels in killing bacteria, viruses, and fungi on countertops and cutting boards while being safer for colored fabrics and less corrosive on metals. Both agents are essential for household cleaning, but hydrogen peroxide offers a gentler alternative for delicate surfaces and non-porous materials.
Safety Precautions and Toxicity Concerns
Bleach contains sodium hypochlorite, which releases toxic chlorine gas when mixed with acids or ammonia, posing significant respiratory risks and skin irritation, requiring careful storage and ventilation during use. Hydrogen peroxide breaks down into water and oxygen, making it less toxic and a safer alternative; however, concentrated forms can cause skin burns and eye damage, necessitating protective gloves and eyewear. Both require avoidance of ingestion and proper dilution, but hydrogen peroxide's lower environmental impact makes it preferable for households prioritizing safety and ecological concerns.
Environmental Impact: Bleach vs Hydrogen Peroxide
Bleach releases harmful chlorine compounds into water systems, contributing to toxic byproducts that damage aquatic ecosystems and persist in the environment. Hydrogen peroxide breaks down into water and oxygen, making it a safer, biodegradable option with minimal ecological footprint. Selecting hydrogen peroxide over bleach reduces chemical pollution and supports sustainable cleaning practices.
Stain Removal Capabilities Compared
Bleach offers powerful stain removal by breaking down tough pigments and disinfecting surfaces, making it ideal for whitening whites and removing mold. Hydrogen peroxide acts as a mild bleaching agent, effectively removing stains on delicate fabrics and surfaces without harsh chemicals. Both agents excel in stain removal, with bleach suited for heavy-duty cleaning and hydrogen peroxide preferred for safer, eco-friendly applications.
Compatibility with Surfaces and Materials
Bleach is highly effective on non-porous surfaces like tile and glass but can cause discoloration and corrosion on fabrics, metals, and certain plastics. Hydrogen peroxide is gentler, safe for most surfaces including carpets and upholstery, and decomposes into water and oxygen, minimizing residue. Selecting the appropriate disinfectant depends on the surface's chemical tolerance and desired cleaning intensity.
Cost and Availability in Stores
Bleach is widely available in most grocery and hardware stores at a relatively low cost, making it an economical choice for many households. Hydrogen peroxide, while also accessible, tends to be slightly more expensive and is often found in pharmacies or specialty cleaning aisles. Both products are generally easy to obtain, but bleach offers a cost advantage and broader retail presence compared to hydrogen peroxide.
Choosing the Right Disinfectant for Your Needs
Bleach offers powerful disinfectant properties effective against a wide range of pathogens, making it ideal for heavy-duty cleaning in bathrooms and kitchens. Hydrogen peroxide provides a safer alternative with strong antimicrobial effects and less toxic residue, suitable for delicate surfaces and food-contact areas. Selecting the right disinfectant depends on surface type, required disinfecting strength, and safety considerations to ensure effective and appropriate sanitation.
Bleach vs Hydrogen Peroxide Infographic
 productdif.com
productdif.com