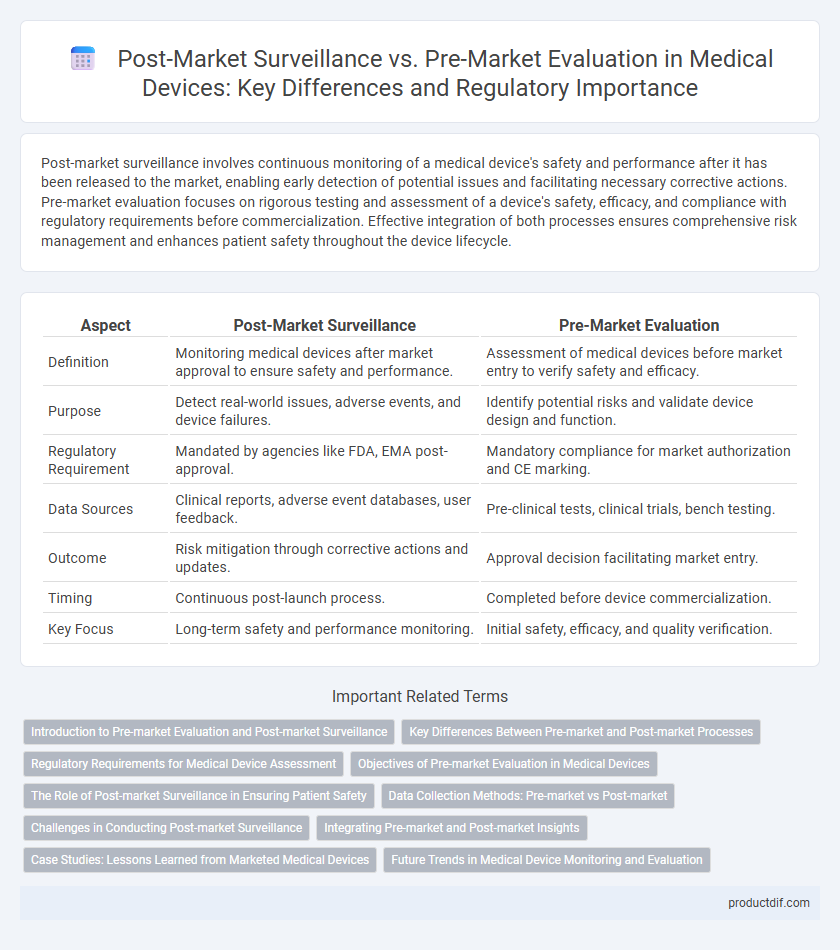
Post-market surveillance involves continuous monitoring of a medical device's safety and performance after it has been released to the market, enabling early detection of potential issues and facilitating necessary corrective actions. Pre-market evaluation focuses on rigorous testing and assessment of a device's safety, efficacy, and compliance with regulatory requirements before commercialization. Effective integration of both processes ensures comprehensive risk management and enhances patient safety throughout the device lifecycle.
Table of Comparison
| Aspect | Post-Market Surveillance | Pre-Market Evaluation |
|---|---|---|
| Definition | Monitoring medical devices after market approval to ensure safety and performance. | Assessment of medical devices before market entry to verify safety and efficacy. |
| Purpose | Detect real-world issues, adverse events, and device failures. | Identify potential risks and validate device design and function. |
| Regulatory Requirement | Mandated by agencies like FDA, EMA post-approval. | Mandatory compliance for market authorization and CE marking. |
| Data Sources | Clinical reports, adverse event databases, user feedback. | Pre-clinical tests, clinical trials, bench testing. |
| Outcome | Risk mitigation through corrective actions and updates. | Approval decision facilitating market entry. |
| Timing | Continuous post-launch process. | Completed before device commercialization. |
| Key Focus | Long-term safety and performance monitoring. | Initial safety, efficacy, and quality verification. |
Introduction to Pre-market Evaluation and Post-market Surveillance
Pre-market evaluation involves rigorous testing, clinical trials, and regulatory review to ensure medical devices meet safety and efficacy standards before market entry. Post-market surveillance continuously monitors device performance through real-world data, adverse event reporting, and periodic safety updates to identify potential risks or failures after commercialization. Combining these processes enhances patient safety and supports regulatory compliance throughout the device lifecycle.
Key Differences Between Pre-market and Post-market Processes
Pre-market evaluation focuses on assessing the safety, efficacy, and regulatory compliance of medical devices before they reach the market, involving clinical trials, risk analysis, and certification processes. Post-market surveillance monitors the device's real-world performance, detecting adverse events, long-term risks, and ensuring ongoing compliance with standards like ISO 13485 and FDA regulations. Key differences include the timing of assessment--pre-market is proactive and validation-based, while post-market is reactive and surveillance-driven--highlighting continuous quality management throughout the device lifecycle.
Regulatory Requirements for Medical Device Assessment
Post-market surveillance monitors medical device performance and safety after market entry, ensuring compliance with ongoing regulatory requirements such as adverse event reporting and periodic safety updates. Pre-market evaluation involves rigorous assessment of clinical data, risk analysis, and conformity with standards like ISO 13485 and FDA 510(k) clearance before device approval. Regulatory agencies mandate these processes to maintain device efficacy, minimize patient risk, and support continuous product improvement.
Objectives of Pre-market Evaluation in Medical Devices
Pre-market evaluation in medical devices aims to ensure safety, effectiveness, and regulatory compliance before market entry by thoroughly assessing device design, performance, and potential risks. This process involves clinical trials, technical documentation, and risk-benefit analysis to identify and mitigate hazards early. Robust pre-market evaluation minimizes post-market surveillance issues, reducing adverse events and enhancing patient safety.
The Role of Post-market Surveillance in Ensuring Patient Safety
Post-market surveillance plays a critical role in ensuring patient safety by continuously monitoring the performance and adverse events of medical devices after they have been released to the market. Unlike pre-market evaluation, which assesses safety and efficacy based on clinical trials and laboratory testing, post-market surveillance captures real-world data reflecting diverse patient populations and long-term device use. This ongoing process enables regulatory bodies and manufacturers to identify risks, implement corrective actions, and improve device safety standards over time.
Data Collection Methods: Pre-market vs Post-market
Pre-market evaluation relies heavily on controlled clinical trials and laboratory testing to gather safety and efficacy data before device approval. Post-market surveillance utilizes real-world evidence from registries, electronic health records, adverse event reports, and user feedback to continuously monitor device performance. Data collection methods during post-market phases emphasize long-term safety monitoring and identification of rare adverse events unavailable in pre-market studies.
Challenges in Conducting Post-market Surveillance
Post-market surveillance in medical devices faces challenges such as data collection inconsistencies, underreporting of adverse events, and difficulties in long-term risk assessment compared to pre-market evaluation. Unlike pre-market evaluation, which relies on controlled clinical trials and standardized testing, post-market surveillance must analyze real-world data with variable quality and completeness. Regulatory bodies emphasize improving traceability and real-time monitoring to address these complexities and ensure ongoing patient safety.
Integrating Pre-market and Post-market Insights
Integrating pre-market evaluation and post-market surveillance in medical device development enables continuous safety and performance improvements by leveraging real-world data alongside initial clinical trial results. Combining these insights enhances risk management strategies and supports regulatory compliance throughout the product lifecycle. This approach fosters proactive identification of potential issues, optimizing device efficacy and patient outcomes over time.
Case Studies: Lessons Learned from Marketed Medical Devices
Case studies of marketed medical devices highlight critical differences between post-market surveillance and pre-market evaluation in ensuring device safety and efficacy. Pre-market evaluation primarily focuses on controlled clinical trials and regulatory approvals to identify potential risks before market entry, while post-market surveillance gathers real-world data to monitor device performance and detect adverse events over time. Lessons learned emphasize the importance of robust post-market surveillance systems to promptly address unforeseen issues and enhance patient safety beyond initial evaluations.
Future Trends in Medical Device Monitoring and Evaluation
Post-market surveillance leverages real-world data and advanced analytics to identify device performance and safety issues beyond controlled pre-market evaluations. Integration of artificial intelligence and wearable technology enables continuous monitoring, enhancing early detection of adverse events and device malfunctions. Future trends emphasize interconnected health ecosystems and regulatory frameworks that incorporate real-time data to improve patient outcomes and device lifecycle management.
Post-market surveillance vs Pre-market evaluation Infographic
 productdif.com
productdif.com