Hypochlorite solution is a powerful disinfectant known for its ability to kill a wide range of pathogens quickly by releasing chlorine, making it ideal for sanitizing surfaces in healthcare and food preparation areas. Quaternary ammonium compounds (quats) offer effective antimicrobial properties with lower corrosiveness and longer-lasting residual effects, suitable for everyday cleaning tasks and surfaces sensitive to harsh chemicals. Choosing between hypochlorite and quats depends on the specific cleaning requirements, surface compatibility, and desired speed and duration of disinfection.
Table of Comparison
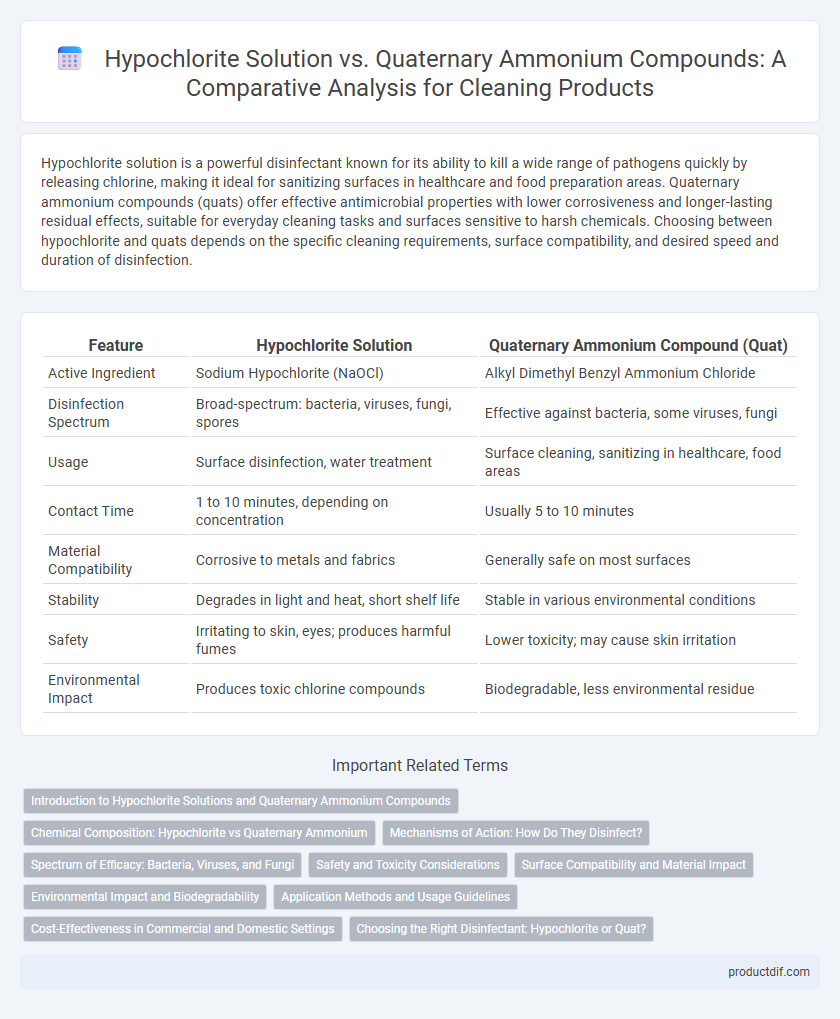
| Feature | Hypochlorite Solution | Quaternary Ammonium Compound (Quat) |
|---|---|---|
| Active Ingredient | Sodium Hypochlorite (NaOCl) | Alkyl Dimethyl Benzyl Ammonium Chloride |
| Disinfection Spectrum | Broad-spectrum: bacteria, viruses, fungi, spores | Effective against bacteria, some viruses, fungi |
| Usage | Surface disinfection, water treatment | Surface cleaning, sanitizing in healthcare, food areas |
| Contact Time | 1 to 10 minutes, depending on concentration | Usually 5 to 10 minutes |
| Material Compatibility | Corrosive to metals and fabrics | Generally safe on most surfaces |
| Stability | Degrades in light and heat, short shelf life | Stable in various environmental conditions |
| Safety | Irritating to skin, eyes; produces harmful fumes | Lower toxicity; may cause skin irritation |
| Environmental Impact | Produces toxic chlorine compounds | Biodegradable, less environmental residue |
Introduction to Hypochlorite Solutions and Quaternary Ammonium Compounds
Hypochlorite solutions, commonly known as bleach, are powerful disinfectants with strong oxidizing properties effective against a broad spectrum of pathogens, including bacteria, viruses, and fungi. Quaternary ammonium compounds (quats) are cationic surfactants that disrupt microbial cell membranes, providing long-lasting antimicrobial activity and residual surface protection. Both are widely used in cleaning products, with hypochlorite favored for rapid, high-level disinfection and quaternary ammonium compounds preferred for routine sanitization and material compatibility.
Chemical Composition: Hypochlorite vs Quaternary Ammonium
Hypochlorite solution primarily contains sodium hypochlorite (NaOCl), a powerful oxidizing agent effective in breaking down organic matter and disinfecting surfaces. Quaternary ammonium compounds consist of nitrogen-based cations with alkyl groups that disrupt cell membranes, providing antimicrobial activity without the strong oxidizing effects of hypochlorite. The chemical composition differences influence their cleaning efficacy, with hypochlorite excelling in bleach-based disinfection and quaternary ammonium favored for surface sanitization and residual antimicrobial protection.
Mechanisms of Action: How Do They Disinfect?
Hypochlorite solution disinfects by releasing free chlorine, which penetrates microbial cell walls, oxidizes essential cellular components, and rapidly destroys viruses, bacteria, and fungi. Quaternary ammonium compounds disrupt microbial membranes by interacting with phospholipids and proteins, leading to leakage of cellular contents and eventual cell death. The oxidative burst of hypochlorite provides broad-spectrum and fast-acting disinfection, while quaternary ammonium compounds offer targeted membrane disruption with residual antimicrobial effects.
Spectrum of Efficacy: Bacteria, Viruses, and Fungi
Hypochlorite solution demonstrates a broad-spectrum efficacy, effectively eliminating bacteria, viruses, and fungi, with strong oxidizing properties that disrupt microbial cell walls and viral envelopes. Quaternary ammonium compounds are particularly effective against gram-positive bacteria and enveloped viruses but show reduced activity against non-enveloped viruses and certain fungal spores. Choosing between these disinfectants depends on the targeted pathogens, making hypochlorite suitable for comprehensive microbial control and quaternary ammonium compounds preferable for targeted bacterial and viral sanitization.
Safety and Toxicity Considerations
Hypochlorite solutions pose significant safety risks due to their corrosive nature and potential to release harmful chlorine gas when mixed with acids, necessitating careful handling and storage protocols. Quaternary ammonium compounds generally exhibit lower acute toxicity and better material compatibility but can cause skin and respiratory irritation with prolonged exposure. Selecting between these disinfectants requires balancing effective microbial eradication with minimizing health hazards and environmental impact.
Surface Compatibility and Material Impact
Hypochlorite solution is highly effective for disinfecting but can cause corrosion and discoloration on metals, plastics, and painted surfaces, limiting its use on delicate materials. Quaternary ammonium compounds offer broader surface compatibility, safely disinfecting without damaging most plastics, rubber, and metals, making them ideal for sensitive or mixed-material environments. Choosing between these disinfectants depends on balancing microbial control needs with the preservation of surface integrity.
Environmental Impact and Biodegradability
Hypochlorite solution, commonly known as bleach, releases chlorine compounds that can form harmful byproducts affecting aquatic ecosystems and exhibit limited biodegradability. Quaternary ammonium compounds (quats) have moderate biodegradability but can accumulate in water systems, posing risks to aquatic life due to their toxicity and persistence. Selecting cleaning products requires balancing effective disinfection with minimizing environmental impact, favoring formulations with safer degradation profiles.
Application Methods and Usage Guidelines
Hypochlorite solution is commonly applied through dilution and surface wiping or mopping, ideal for disinfecting hard non-porous surfaces but requiring careful handling due to its corrosive nature. Quaternary ammonium compounds (quats) are typically used as ready-to-use sprays or wipes, effective on a broader range of surfaces including plastics and fabrics, with less corrosive impact and longer residue activity. Proper usage guidelines for hypochlorite emphasize avoiding mixing with ammonia or acids, while quaternary ammonium compounds require thorough surface contact time to ensure microbial efficacy.
Cost-Effectiveness in Commercial and Domestic Settings
Hypochlorite solution offers a lower upfront cost and effective broad-spectrum disinfection, making it a cost-effective choice for high-traffic commercial and domestic settings. Quaternary ammonium compounds, while generally more expensive, provide longer-lasting antimicrobial effects and surface compatibility that reduce the need for frequent reapplication. Evaluating total cost of use including product price, frequency of application, and surface safety helps determine the optimal disinfectant choice for specific cleaning needs.
Choosing the Right Disinfectant: Hypochlorite or Quat?
Hypochlorite solution offers broad-spectrum antimicrobial efficacy and rapid action, making it ideal for high-risk areas requiring fast and thorough disinfection. Quaternary ammonium compounds (Quats) provide longer-lasting residual activity and are less corrosive, suited for routine cleaning in hospitals and foodservice settings. Selecting the right disinfectant depends on surface compatibility, target pathogens, and the specific cleaning environment's safety requirements.
Hypochlorite Solution vs Quaternary Ammonium Compound Infographic
 productdif.com
productdif.com