Fermentation is a natural process where yeast or bacteria convert sugars into alcohol and carbon dioxide, imparting unique flavors and effervescence to beverages like beer and kombucha. Carbonation, on the other hand, involves artificially dissolving carbon dioxide into a liquid, often enhancing the beverage's crispness and fizziness without altering its base flavor. Understanding the difference between fermentation and carbonation helps consumers appreciate the complexity and production methods behind their favorite drinks.
Table of Comparison
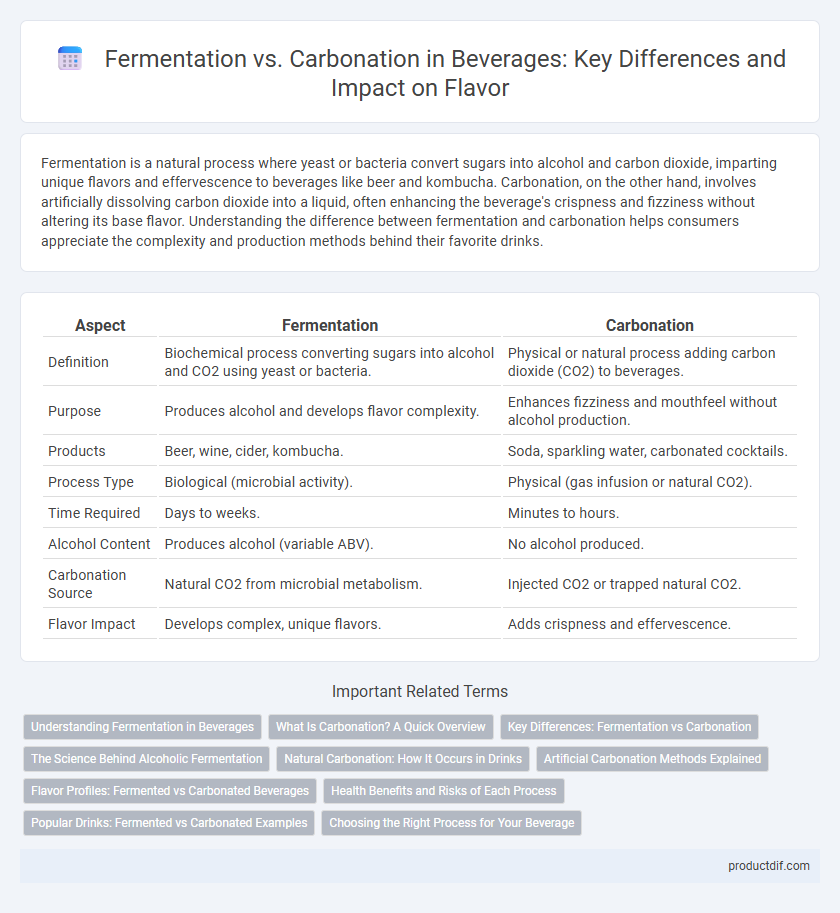
| Aspect | Fermentation | Carbonation |
|---|---|---|
| Definition | Biochemical process converting sugars into alcohol and CO2 using yeast or bacteria. | Physical or natural process adding carbon dioxide (CO2) to beverages. |
| Purpose | Produces alcohol and develops flavor complexity. | Enhances fizziness and mouthfeel without alcohol production. |
| Products | Beer, wine, cider, kombucha. | Soda, sparkling water, carbonated cocktails. |
| Process Type | Biological (microbial activity). | Physical (gas infusion or natural CO2). |
| Time Required | Days to weeks. | Minutes to hours. |
| Alcohol Content | Produces alcohol (variable ABV). | No alcohol produced. |
| Carbonation Source | Natural CO2 from microbial metabolism. | Injected CO2 or trapped natural CO2. |
| Flavor Impact | Develops complex, unique flavors. | Adds crispness and effervescence. |
Understanding Fermentation in Beverages
Fermentation in beverages is a biochemical process where yeast or bacteria convert sugars into alcohol, carbon dioxide, and other compounds, creating distinct flavors and natural carbonation. This natural fermentation results in complex taste profiles found in beers, wines, and kombuchas, enhancing their sensory appeal. Understanding fermentation is essential for controlling alcohol content, flavor development, and beverage texture.
What Is Carbonation? A Quick Overview
Carbonation is the process of dissolving carbon dioxide gas into a beverage, creating bubbles and a fizzy texture. It can occur naturally through fermentation or be artificially added by injecting CO2 under pressure. This process enhances the sensory experience by adding a crisp, refreshing mouthfeel and can also help preserve the drink.
Key Differences: Fermentation vs Carbonation
Fermentation is a biochemical process where yeast and bacteria convert sugars into alcohol and carbon dioxide, producing natural carbonation and unique flavors in beverages like beer and wine. Carbonation, in contrast, refers to the artificial or natural introduction of carbon dioxide into a beverage to create bubbles without altering its alcohol content or flavor profile. Understanding these key differences is crucial for beverage production, as fermentation influences both flavor complexity and alcohol levels, while carbonation primarily impacts texture and mouthfeel.
The Science Behind Alcoholic Fermentation
Alcoholic fermentation is a metabolic process where yeast converts sugars, primarily glucose, into ethanol and carbon dioxide under anaerobic conditions. This biochemical reaction involves enzymes like zymase that break down carbohydrates, releasing energy and producing alcohol as a byproduct. Unlike carbonation, which adds dissolved carbon dioxide for effervescence, fermentation naturally generates alcohol alongside carbon dioxide, influencing both flavor and beverage texture.
Natural Carbonation: How It Occurs in Drinks
Natural carbonation occurs during fermentation when yeast consumes sugars and produces carbon dioxide as a byproduct, which dissolves into the beverage, creating bubbles. This process is common in traditional drinks like champagne, kombucha, and certain craft beers, where the carbonation develops organically over time. The natural carbonation imparts a subtle effervescence and complex flavor profile that distinguishes these beverages from those artificially carbonated.
Artificial Carbonation Methods Explained
Artificial carbonation methods involve injecting carbon dioxide (CO2) directly into beverages under controlled pressure to create effervescence, a process widely used in soft drinks and sparkling water production. Unlike natural fermentation, which produces carbonation as a byproduct of yeast metabolizing sugars into alcohol and CO2, artificial carbonation allows precise control over carbonation levels, ensuring consistent taste and fizziness. Techniques such as forced carbonation in sealed tanks and carbonation stones are common industrial practices that enhance efficiency and scalability in beverage manufacturing.
Flavor Profiles: Fermented vs Carbonated Beverages
Fermented beverages develop complex flavor profiles through the biochemical activity of yeast and bacteria, producing natural acids, alcohol, and esters that result in rich, tangy, and sometimes sour notes. Carbonated beverages rely on the infusion of carbon dioxide gas, which creates a sharp, effervescent texture and a cleaner, often sweeter taste, with minimal alteration to the base liquid's original flavor. The dynamic depth in fermented drinks contrasts with the bright, crisp sensations typical of carbonated options, influencing consumer preferences based on taste complexity and mouthfeel.
Health Benefits and Risks of Each Process
Fermentation produces natural probiotics and beneficial enzymes that support gut health and improve digestion, while the organic acids and antioxidants formed during fermentation offer immune-boosting properties. Carbonation involves dissolving carbon dioxide into beverages, which can cause bloating and discomfort in sensitive individuals but does not contribute additional nutrients or probiotics. Understanding the distinct health impacts of fermented versus carbonated drinks aids consumers in making informed choices based on digestive health and nutritional benefits.
Popular Drinks: Fermented vs Carbonated Examples
Fermented beverages such as beer, wine, and kombucha rely on natural yeast and bacteria to convert sugars into alcohol and organic acids, creating complex flavors and probiotic benefits. Carbonated drinks like soda, sparkling water, and club soda undergo carbonation, a process where carbon dioxide gas is dissolved under pressure, producing effervescence without fermentation. Popular fermented drinks typically offer alcohol content and unique taste profiles, while carbonated beverages appeal with their refreshing fizz and non-alcoholic varieties.
Choosing the Right Process for Your Beverage
Fermentation naturally produces carbonation through the metabolic activity of yeast converting sugars into alcohol and carbon dioxide, making it ideal for beverages like beer and kombucha with complex flavor profiles. Carbonation, often achieved by injecting CO2 gas, offers precise control over fizziness in sodas and sparkling waters, ensuring consistent taste and mouthfeel. Selecting fermentation or carbonation depends on desired beverage characteristics, production scale, and flavor complexity.
Fermentation vs Carbonation Infographic
 productdif.com
productdif.com